INTRODUCTION
According to the World Health Organization burden of disease from ambient air pollution reports, air pollution, especially that caused by particulate matter (PM), is a risk factor associated with acute lower respiratory infections, chronic obstructive pulmonary disease, lung cancer, ischemic heart disease (IHD), and stroke [
1,
2]. Ambient fine PM (PM
2.5) air pollution caused 7.6% of total mortality and contributed to 17.1% of IHD mortality in 2015 [
1]. Several mechanisms assessing the association between PM and cardiovascular disease have been proposed, but the association between PM and pulse wave velocity (PWV), which is an independent predictor of cardiovascular outcome, has not been fully investigated in the healthy population. One possible mechanism is that PM could be associated with endothelial dysfunction, accelerate acute arterial vasoconstriction in healthy adults, and eventually disrupt systolic function and increase heart rate variability [
3].
Aortic PWV is an independent predictor of cardiovascular morbidity and mortality in patients with end-stage renal disease and essential hypertension as well as in the general population [
4]. In addition, brachial-ankle PWV (baPWV) is an accurate and noninvasive indicator of aortic PWV and has been proven suitable for screening vascular damage in a large cohort [
5]. Among several studies evaluating the effects of PM on arterial stiffness, three studies focused on the effect of PM
10. Adamopoulos et al. [
6] analyzed atmospheric pollution variables, including atmospheric PM concentrations, and their effects on arterial stiffness. They showed that 24-hour mean PM
10 levels were associated with an increased amplitude of the aortic-wave reflection magnitude (augmentation pressure) in patients with hypertension but not with PWV. In a cross-sectional study of 127 patients undergoing hemodialysis with a mean baPWV of 1,767 cm/s, Weng et al. [
3] reported that the 12-month average PM
10 concentration (57.9┬▒5.7 ╬╝g/m
3) was associated with elevated baPWV. Ljungman et al. [
7] showed that living near a major road was associated with higher arterial stiffness. However, satellite measurements of PM levels were not associated with arterial stiffness in the healthy Framingham Heart Study population, and no associations were observed with short-term exposure to air pollutants such as PM
2.5, black carbon, sulfate, nitrogen oxides, and ozone.
Therefore, this study aimed to determine whether the correlation between long- and short-term exposure to PM10 and baPWV exists in apparently healthy cancer-free adults aged 40 years and older and to identify susceptible subgroups.
METHODS
1. Study Population
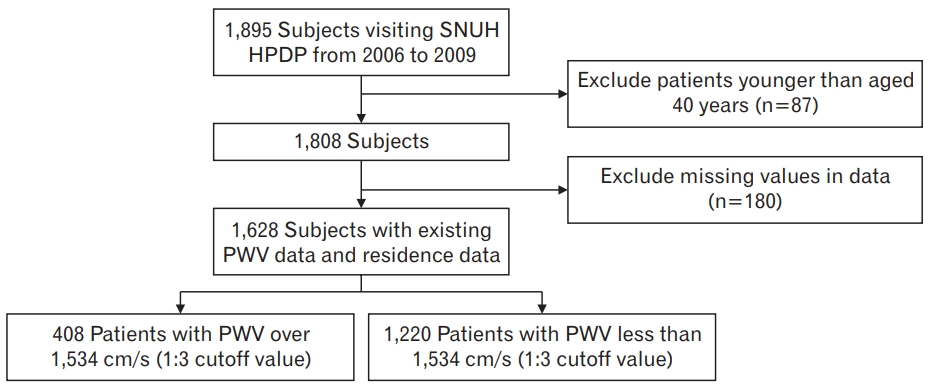
We initially investigated cancer-free patients who underwent medical screening examinations at Seoul National University Health Promotion Center between January 1, 2006, and December 31, 2009. Since the reference values of baPWV are influenced by age, participants aged 40 years and older were included in this study. Medical and demographic data were collected from chart reviews and online databases at our hospital. Patients with missing variables, such as blood pressure, fasting blood glucose, cholesterol levels (including high-density lipoprotein [HDL] cholesterol and triglycerides), waist circumference, body mass index (BMI), smoking status, drinking status, regular exercise, residence location, baPWV measurements, and antihypertensive and antidiabetic medication status were excluded from the study (
Figure 1).
This study was conducted in accordance with the tenets of the Declaration of Helsinki and the need to obtain patient consent was waived by the Seoul National University Hospital Institutional Review Board (IRB approval no., E-1803-108-932).
2. Major Exposure Variables
Air pollution levels were recorded using a network of 265 monitoring stations near the patientsŌĆÖ living areas in South Korea. Data on air quality status obtained by the Korean Ministry of Environment were used [
8]. For PM
10 values, the 24-hour average concentrations of PM on the day of medical screening and moving averages of the prior 1ŌĆō3 days (PM
10 Lag 0 to PM
10 Lag 0ŌĆō3) were used to evaluate the effect of short-term exposure to PM
10 on health outcomes. Long-term exposure to PM
10 was examined using a 365-day moving average of PM
10 concentrations (PM
10 Lag 0ŌĆō365). If there was no monitoring station in a participantŌĆÖs residential district, we selected the pollution level from the nearest station.
3. Covariates
Hypertension and diabetes status, including the medication details, were ascertained using self-administered questionnaires. Patients were classified as nonsmokers, ex-smokers, and current smokers using self-administered structured questionnaires, which were rechecked by trained nurses. Heavy alcohol consumption was defined as alcohol consumption of more than 30 g/d (210 g/wk) [
9]. Concise methods for body composition measurements have been described previously [
10].
4. Definition of Metabolic Syndrome
Metabolic syndrome was defined according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) and American Heart Association/National Heart Lung and Blood Institute (AHA/NHLBI) [
11,
12]. The abdominal obesity criterion was modified according to Asian guidelines [
13]. As a result, patients who met three or more of the following criteria were selected: (1) waist circumference Ōēź90 cm for men or Ōēź80 cm for women; (2) triglyceride levels of 150 mg/dL or higher; (3) HDL cholesterol levels less than 40 mg/dL in men or less than 50 mg/dL in women; (4) blood pressure levels of 130/85 mm Hg or higher; and (5) elevated fasting blood sugar levels of 100 mg/dL or higher.
5. Brachial-Ankle Pulse Wave Velocity Measurement
BaPWV was measured using a Vascular Profiler 1000 (VP-1000; Omron Healthcare, Kyoto, Japan), with blood pressure cuffs placed around the arms and legs in the supine position, and was calculated using the equation: baPWV=(D1-D2)/t, where D1 is the distance between the heart and ankle, D2 is the distance between the heart and brachium, and t is the transit time between brachial arterial waves and tibial arterial waves. The baPWV was measured by a trained technician.
6. Statistical Analysis
Previously, a baPWV cutoff value of 1,547 cm/s, which is close to the highest quartile value of the outcome variable of baPWV (=1,534 cm/s) in this study, has been suggested as an independent predictor of asymptomatic coronary artery disease evaluated by coronary computed tomography angiography. Considering previous studies, we used a cutoff value of 1,534 cm/s for our data analysis.
Subjects were categorized into two groups: those less than the third quartile and those greater than or equal to the third quartile of baPWV (1,534 cm/s, 75th percentile). P-values were calculated using the t-test for continuous variables and chi-square test for categorical variables in the baPWV subgroups (< or Ōēź1,534 cm/s). Univariate and multivariate logistic regression analyses were performed using a baPWV cutoff value of 1,534 cm/s as an outcome variable and PM10 as exposure variables (short- and long-term), including age, sex, the use of antihypertensive medication and anti-diabetic medication, systolic blood pressure, daily mean temperature, year of examination, and residence area of participants as covariates. An effect modification analysis was conducted to investigate vulnerable subgroups by including an interaction term of PM10 exposure and a subgroup in addition to the covariates in the main models. The odds ratios (ORs) and 95% confidence intervals were estimated. Statistical analysis was performed using Stata ver. 16.0 (Stata Corp., College Station, TX, USA).
RESULTS
A total of 1,628 patients were eligible for the study, as shown in
Figure 1.
Table 1 shows the baseline characteristics of the study population stratified according to a baPWV cutoff of 1,534 cm/s. The mean┬▒standard deviation baPWV and age of the total study population were 1,419┬▒244 cm/s and 56.0┬▒8.4 years, respectively.
In the study population, age, hypertensive medication, diabetes mellitus medication status, blood pressure (both systolic and diastolic), and glucose levels were statistically different between the high (Ōēź1,534) and low (<1,534) baPWV groups. More specifically, patients with high baPWV were mostly older, were more likely to take hypertensive medication, had higher blood pressure, used more diabetes medication, and had higher glucose levels. There were no statistically significant differences between PM
10 levels at Lag 0 to Lag 3 days and in moving averages (
Table 1).
Table 2 also shows the statistically significant associations between age, systolic and diastolic blood pressure, triglyceride levels, glucose levels, and high baPWV on unadjusted logistic regression analysis. However, we did not observe a significant relationship between short- and long-term exposure to PM
10 and high baPWV in the total study population. Although there were no significant associations between long-term exposure to PM
10 and high baPWV in the subgroups (
Table 3), the associations between short-term exposure to PM
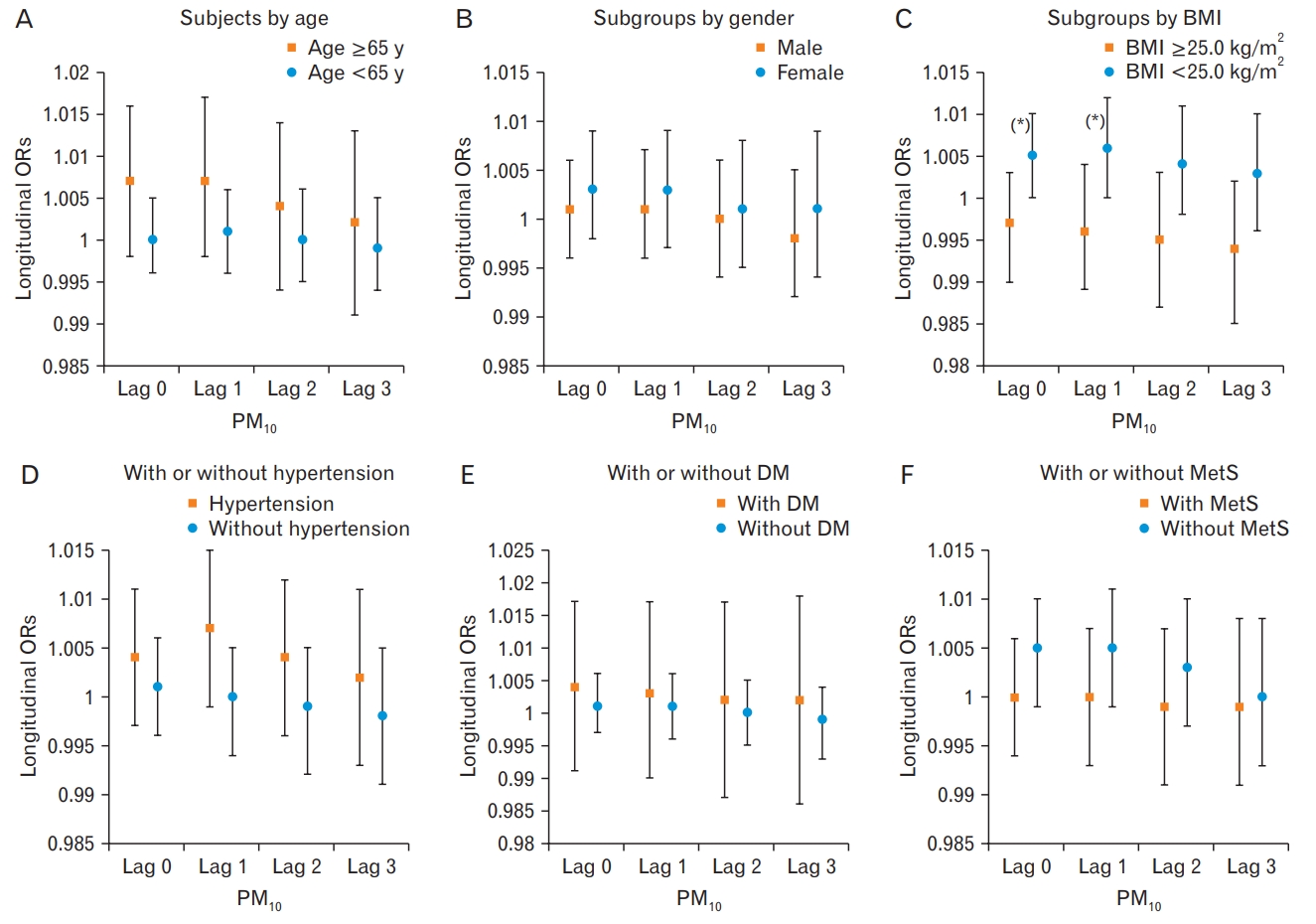
10 and high baPWV were marginally significant in non-obese subjects (
Figure 2). A 10-╬╝g increase in the 24-hour moving average at Lag 0ŌĆō1 PM
10 exposure was associated with a 6% increased risk of high baPWV in non-obese subjects (OR, 1.059; P=0.058). The elderly, non-obese, and hypertensive individuals showed statistically non-significantly higher ORs than that did their counterparts (the young, obese and non-hypertensive individuals) during short-term exposure to PM
10 in Lag 0ŌĆō1. When we conducted stratified analysis to determine the differential effects of short-term exposure to PM
10 exposure of Lag 0 to Lag 0ŌĆō3 on high PWV, an unfavorable effect was more prominent in the non-obese group than in the obese group (P-value for interaction PM
10 Lag 0, 0.041; PM
10 Lag 0ŌĆō1, 0.038) (
Supplementary Table 1,
Figure 2).
DISCUSSION
When we assessed the short-term PM10 exposure through the 24-hour moving average of Lag 0 to Lag 3 days prior to medical examination, we found no statistically significant associations in the total study population and subgroups. When we investigated the subgroups according to age, sex, BMI, anti-hypertensive and anti-diabetes medication status, and metabolic syndrome with short-term exposure to PM10 from Lag 0 to Lag 3, participants with a lower BMI (<25.0 kg/m2) presented a slightly greater risk of high PWV compared to their counterparts with a moving average of Lag 0ŌĆō1. In particular, a 10-╬╝g increase in the 24-hour moving average of PM10 exposure of Lag 0ŌĆō1 was marginally associated with a 6% elevated risk of high baPWV in non-obese subjects. Long-term exposure assessed through a 365-day moving average of PM10 (PM10 Lag 0ŌĆō365) was not associated with an increased risk of high baPWV among all subjects and subgroups.
Previous studies on the association between PM
10 and baPWV showed inconsistent results due to differences in study populations and small sample sizes. In a systematic review, based on the published literature until January 2017, Zanoli et al. [
14] suggested that short- and long-term exposure to gaseous and PM pollutants, including nitric oxide, sulfur dioxide, PM
10, and PM
2.5, may be associated with increased arterial stiffness and wave reflection. As a potential marker of arterial stiffness, parameters such as carotid-femoral PWV, baPWV, and augmentation index were assessed in 11 published studies from 2010 to 2016. Various subgroups such as older adults, urban population, patients with hypertension, and patients undergoing hemodialysis were more sensitive to air pollution variables, as manifested by arterial stiffness [
3,
6,
15,
16]. Among them, only one study conducted by Weng et al. [
3] observed that the 12-month average concentration of PM
10 was associated with elevated baPWV in patients undergoing hemodialysis (╬▓=0.13, P=0.04).
In 2018, in a well-characterized large community-based cohort of the Framingham Heart study [
7], a positive association between living near a major road and higher arterial stiffness was observed. However, no associations were observed with short- and long-term exposure to air pollutants such as the satellite-measured levels of PM
2.5, black carbon, sulfate, nitrogen oxides, and ozone, with arterial stiffness. Recently, an interquartile increase in PM
10 and PM
2.5 did not significantly increase the PWV in 40 healthy volunteers aged 60 years and older in a randomized, crossover study [
17]. Our study also showed no significant associations between short-term and long-term exposure to PM
10 and baPWV in apparently healthy cancer-free subjects. We observed a marginally elevated risk of high PWV associated with short-term exposure to a 24-hour moving average of PM
10 exposure of Lag 0ŌĆō1 in non-obese subjects. This association in non-obese subjects was significantly different from that in obese subjects.
Several mechanisms underlying the association between PM and PWV have been postulated. Mild and transitory inflammatory stimuli have been proposed as possible links between arterial stiffening [
14], functional arterial stiffening, and short-term exposure to air pollutants. Additionally, exposure to PM pollutants is associated with acute arterial vasoconstriction [
18] and endothelial dysfunction [
19]. Long-term exposure to PM for months to years has been associated with atherosclerosis, vascular dysfunction, and eventually subclinical diseases such as high blood pressure, increased carotid intima media thickness, and increased calcification of arteries [
7].
The possible mechanism underlying the differential effect of short-term exposure to PM
10 on high PWV in non-obese subjects may be as follows. Previously, Tang et al. [
20] suggested that obese individuals have greater nutritional reserves and can therefore cope better with acute stressful events and increased metabolic demands due to the beneficial effect of adipose tissue induced by beneficial hormones and cytokines. In our study, relatively healthy, non-obese individuals showed significantly lower body fat percentage based on our data (body fat % of obese versus non-obese=30.4% versus 25.9%), which may be a possible mechanism by which non-obese individuals have difficulty coping with short-term exposure to PM
10, resulting in high PWV. Further research is required to validate our findings.
This study used various laboratory data and clinical measurements to determine specific vulnerable subgroups and explain the potential links underlying the relationship between PM and inflammation, fitness, level of adiposity, and PWV. However, our study had some limitations. Although we analyzed the association between PM10 and baPWV in short- and long-term exposures, we did not analyze PM2.5, because PM2.5 data were only available for the city of Seoul between 2006 and 2009, whereas PM10 was available for the entire nation. The study population we used underwent private health screening tests in a single university hospital; thus, this group may not be representative of the general population. Furthermore, we used both short- and long-term PM exposure assessments with fixed monitoring data, which may cause non-differential misclassification at the individual level of exposure and underestimate the association in long-term exposure-related adverse health effects. Finally, because we did not have information regarding the timing of the activities of each patient, we could not ascertain the exact location and timing of exposure to PM10, which could lead to a bias if the PM10 levels between the patientŌĆÖs workplace and residence differ. Nevertheless, because exposure misclassification could lead to a null hypothesis, we can presume that our results could underestimate the effects of PM10 compared to those that could be obtained by measuring PM10 exposure levels with more precision. In other words, because we did not install 24-hour air pollution monitoring devices for each patient, we could not consider the air pollution levels when commuting to and from work; thus, a more sophisticated modeling approach is required in future studies.
In conclusion, we showed the probable adverse effect of short-term exposure to PM10 on high baPWV in non-obese subjects compared to their obese counterparts. Further large and prospective studies are necessary to validate and elucidate the mechanism underlying of the influence of PM on baPWV, especially in healthy, non-obese subjects.