|
 |
- Search
| Korean J Fam Med > Volume 36(5); 2015 > Article |
Abstract
Background
Small vessel disease is an important cause of cerebrovascular diseases and cognitive impairment in the elderly. There have been conflicting results regarding the relationship between Helicobacter pylori infection and ischemic stroke. This study aimed to examine the association between H. pylori infection and cerebral small vessel disease.
Methods
The study included 1,117 patients who underwent brain magnetic resonance imaging and H. pylori identification between 2005 and 2013 at Health Promotion Center, Seoul National University Hospital. Multivariable logistic regression analysis was used to assess the association between H. pylori infection and small vessel disease with adjustment for age, sex, hypertension, diabetes mellitus, dyslipidemia, body mass index, smoking status, problem drinking, and antiplatelet use.
Results
The adjusted odds ratios (aORs) for the association between H. pylori infection and silent brain infarction and cerebral microbleeds were 1.03 (95% confidence interval [CI], 0.66-1.61) and 0.70 (95% CI, 0.38-1.28), respectively. The aORs for silent brain infarction and cerebral microbleeds were 0.81 (95% CI, 0.44-1.44) and 0.59 (95% CI, 0.30-1.18) in patients aged <65 years and 1.59 (95% CI, 0.78-3.22) and 1.89 (95% CI, 0.38-9.33) in those aged >65 years, respectively. Moreover, the aORs for silent brain infarction and cerebral microbleeds were 0.96 (95% CI, 0.54-1.71) and 0.74 (95% CI, 0.33-1.69) in H. pylori-infected patients without atrophic gastritis and 0.89 (95% CI, 0.48-1.62) and 0.99 (95% CI, 0.43-2.27) in those with atrophic gastritis, respectively.
Small vessel disease refers to all ischemic and hemorrhagic manifestations of the small vessels of the brain.1) In recent years, this condition is being frequently detected in asymptomatic people of all ages because of an increasing number of healthy individuals undergoing brain imaging in routine health check-ups. It is important to determine the risk factors for small vessel disease because it is associated with cerebrovascular diseases and plays an important role in cognitive impairment, psychiatric disorders, and functional loss in the elderly.1) Age and hypertension are known to be strong risk factors for small vessel disease, while other risk factors such as coronary artery disease, metabolic syndrome, diabetes, and renal impairment are also likely to be significant, although these findings are not consistent and require further investigation.2,3,4)
Helicobacter pylori is a gram-negative, spiral bacterium that infects the gastric mucosa and causes various gastrointestinal diseases such as chronic gastritis, peptic ulcers, and gastric cancer.5) The seroprevalence of H. pylori is about 60% in South Korea, which is relatively higher than that in other developed countries.6,7) Chronic inflammation caused by H. pylori infection has been studied by several clinicians because it causes atherosclerosis, which can eventually lead to coronary heart diseases such as myocardial infarction.8,9,10,11,12)
Furthermore, several studies have shown a positive relationship between H. pylori infection and ischemic stroke, suggesting that this infection could be a potential risk factor for ischemic stroke.13,14,15,16,17) However, the underlying mechanism is still unclear, and different studies have reported conflicting results.18,19) To the best of our knowledge, no previous study has assessed the relationship between H. pylori infection and hemorrhagic stroke. Moreover, data on the possible risk of atherosclerosis in the small arterioles of the brain in asymptomatic cerebral small vessel disease patients with H. pylori infection are lacking. Therefore, this study aimed to examine the association between H. pylori infection and cerebral small vessel disease.
Patients who visited the Health Promotion Center in Seoul National University Hospital for a routine health check-up between 2005 and 2013 were reviewed. This study analyzed 1,834 patients who had undergone brain magnetic resonance imaging (MRI) and H. pylori infection assessment by esophagogastroduodenoscopy within 2 months before or after the brain MRI. Of the 1,834 patients, 33 with a history of stroke were excluded. Moreover, patients with missing data on age, sex, body mass index, blood pressure, serum fasting glucose levels, glycated hemoglobin (HbA1c) levels, serum cholesterol levels, smoking status, amount of alcohol intake per week, or current antiplatelet use were excluded from the study. Therefore, 1,117 patients were included in the final analysis. This study was approved by the institutional review board of Seoul National University Hospital (IRB No. 1310-117-532).
Clinical data on age, sex, previously diagnosed diseases, smoking status, amount of alcohol intake, current use of an antihypertensive, hypoglycemic, or lipid-lowering agent, and current use of an antiplatelet were obtained by a trained physician through interviews with the patients. Height, weight, and blood pressure were measured and serum samples were obtained in the fasting state in all patients.
Hypertension was defined as a systolic blood pressure of Ōēź140 mm Hg, diastolic blood pressure of Ōēź90 mm Hg, or current treatment with an antihypertensive medication. Diabetes was defined as a fasting blood glucose level of Ōēź126 mg/dL, HbA1c level of Ōēź6.5%,20) or current treatment with insulin or an oral hypoglycemic medication. Hyperlipidemia was defined as a total cholesterol level of Ōēź240 mg/dL or current treatment with a lipid-lowering medication. Smoking status was classified into 3 categories, namely, never smoker, past smoker, and current smoker. Alcohol intake was described as the number of glasses of alcohol consumed per week, which was further converted to grams of alcohol. Problem drinking was defined as a total alcohol intake of Ōēź40 g/wk for men and Ōēź20 g/wk for women. Current antiplatelet use was recorded as positive if the patient provided a positive or "yes" response to at least one of the questions regarding the current use of aspirin or clopidogrel, and information regarding current antiplatelet use was double-checked by a nurse before esophagogastroduodenoscopy was performed.
H. pylori infection was detected using a rapid urease test or by histologic examination. In the Health Promotion Center in Seoul National University Hospital, the rapid urease test is usually performed in patients with signs of peptic ulcers, suspected gastric cancer, chronic atrophic gastritis, or intestinal metaplasia during the examination or when the patient requests that the test be performed beforehand. Histologic examination is usually performed for suspected precancerous lesions in order to rule out gastric cancer. Helicobacter pylori infection was defined as a positive rapid urease test result or the histologic confirmation of the presence of H. pylori.
Small vessel disease was classified into 2 categories: silent brain infarction (SBI) and cerebral microbleeds (CMB). SBI was defined as the presence of a focal T2-hyperintense lesion >3 mm with low signal intensity on T1-weighted images.21) CMB were defined as tiny, round, dark-signal lesions detected on T2-weighted images or gradient-echo MRI.22)
Baseline characteristics of the study population were compared using the chi-square test for categorical variables and the Student t-test for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of small vessel disease in patients with H. pylori infection were calculated using multivariable logistic regression analysis. Because the rapid urease test and histologic examinations have different sensitivity and specificity for identifying H. pylori infection, a subgroup analysis using the rapid urease test or by histologic examination was performed depending on which method was used for the detection of H. pylori infection. To test the robustness of the results, data were analyzed by age group and by the presence of atrophic gastritis. Statistical analyses were performed using STATA ver. 12.0 (Stata Co., College Station, TX, USA). A P-value of <0.05 in two-tailed tests was considered statistically significant.
Baseline characteristics of the small vessel disease patients and control patients are presented in Table 1. The mean age of the SBI patients was 62.5 years, which was significantly higher than that of the control patients. Prevalence of hypertension was higher in the SBI and CMB patients than in the control patients (57.8% vs. 33.1% and 52.1% vs. 34.3%, respectively). Diabetes mellitus was more common in the SBI and CMB patients than in the control patients (26.7% vs. 15.5% and 29.2% vs. 15.8%, respectively).
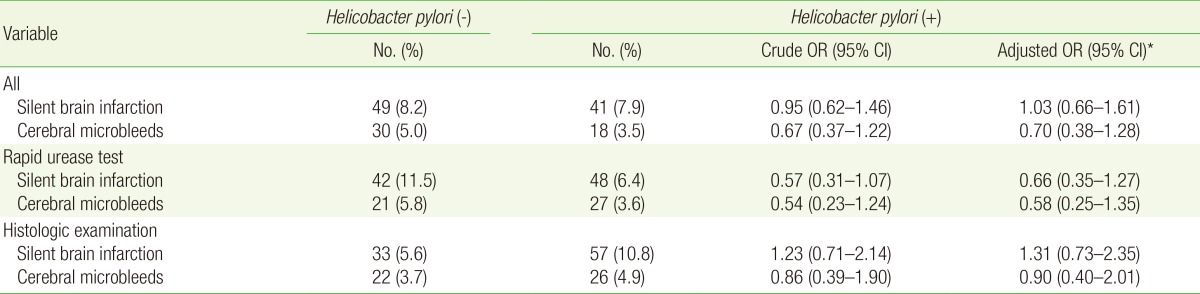
Table 2 shows the adjusted ORs (aORs) for the association between H. pylori infection and small vessel disease. There were no significant differences in the prevalence of SBI and CMB between the H. pylori-infected and control patients after adjustment for the potential risk factors for small vessel disease. The aORs for SBI and CMB in the H. pylori-infected patients were 1.03 (95% CI, 0.66-1.61) and 0.70 (95% CI, 0.38-1.28), respectively. When the analysis was restricted to patients with H. pylori infection confirmed by the rapid urease test, the aORs for SBI and CMB were 0.66 (95% CI, 0.35-1.27) and 0.58 (95% CI, 0.25-1.35), respectively. The aOR for SBI was slightly higher in the H. pylori-infected patients than in the control patients, but this difference was not statistically significant.
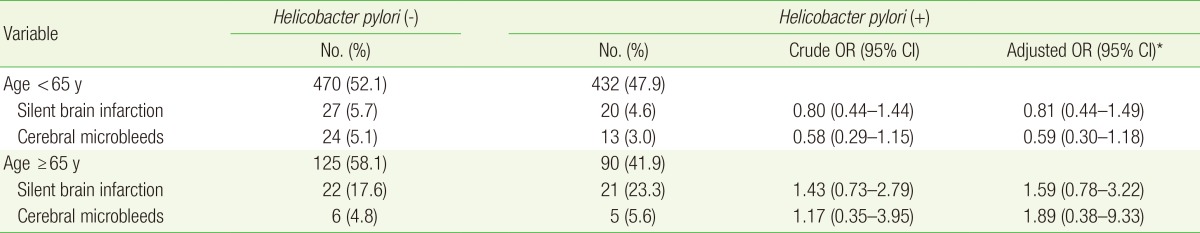
The ORs for small vessel disease in patients of different age groups are shown in Table 3. The aORs for SBI and CMB in patients <65 years of age were 0.81 (95% CI, 0.44-1.44) and 0.59 (95% CI, 0.30-1.18), respectively. The aORs for SBI and CMB in patients >65 years of age were 1.59 (95% CI, 0.78-3.22) and 1.89 (95% CI, 0.38-9.33), respectively, which shows that the results were consistent among the different age groups.
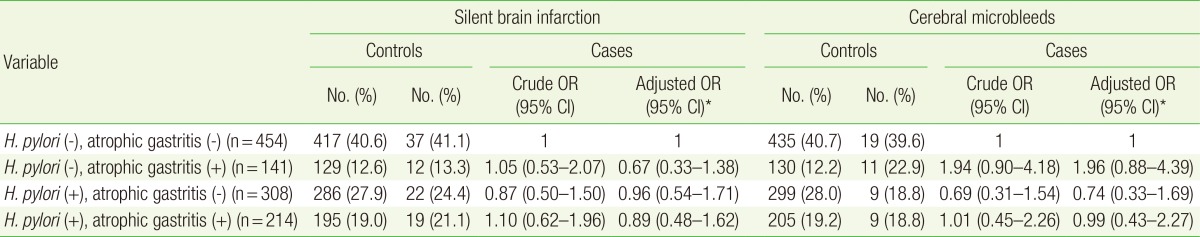
The ORs for small vessel disease in H. pylori-infected patients with and those without atrophic gastritis are shown in Table 4. Of the 1,117 patients, 308 had H. pylori infection without atrophic gastritis and 214 had H. pylori infection with signs of atrophic gastritis on endoscopic examination. The aORs for SBI and CMB in H. pylori-infected patients without atrophic gastritis were 0.96 (95% CI, 0.54-1.71) and 0.74 (95% CI, 0.33-1.69), respectively. The results were consistent in H. pylori-infected patients with signs of atrophic gastritis with aORs of 0.89 (95% CI, 0.48-1.62) and 0.99 (95% CI, 0.43-2.27) for SBI and CMB, respectively.
In this study, no association was found between H. pylori infection and small vessel disease after adjustment for potentially confounding variables. We assumed that old age could affect the risk of small vessel disease, but no such association was observed in patients aged under and over 65 years. Because not all patients who underwent esophagogastroduodenoscopy underwent the rapid urease test or histologic examination, the presence of atrophic gastritis could have been a confounding risk factor. However, the results were not significant regardless of the presence of atrophic gastritis on endoscopic examination.
To the best of our knowledge, this study is the first to examine the association between H. pylori infection and small vessel disease; assuming that there is a similar pathological pathway between small vessel disease and ischemic stroke, we referred to previous studies on the association between H. pylori infection and ischemic stroke. According to the previous studies, the relationship between H. pylori infection and stroke is controversial. In a case-control study, H. pylori seropositivity was higher in symptomatic cerebrovascular disease patients than in the control patients, especially in the case of the lacunar stroke subtype,15) but these results were not consistent with our results. Moreover, in this study, because spouses were assigned to the control group, the subjects were not well matched in terms of factors such as smoking status and underlying disease. Yang et al.18) performed a case-control study with 150 ischemic patients and 131 controls and reported no significant relationship between H. pylori infection and ischemic stroke. There was no significant association between H. pylori infection and the stroke subgroups including the small artery disease group. Sawayama et al.17) reported that ischemic stroke due to small artery occlusion was associated with chronic H. pylori infection. These conflicting results of the different studies reflect differences in the sample size, study population, H. pylori prevalence rate, and study design between the studies, but mostly the difference between ischemic stroke and small vessel disease. There are pathological differences between lacunar infarction, a type of ischemic stroke, and small vessel disease.23) Moreover, a high prevalence of H. pylori infection in Asian countries may lead to different results between Asian and non-Asian studies. Finally, selection bias could have affected the results because not all patients who underwent esophagogastroduodenoscopy underwent H. pylori infection assessment.
One of the strengths of our study is that we used the rapid urease test and histologic examination, which are the gold standards for the identification of current H. pylori infection. Most previous studies used serum antibody tests for detecting H. pylori infection, which are less appropriate because the findings do not necessarily indicate current infection as patients who have been previously treated for this infection can also test positive. Moreover, the sample size in our study was larger than that in the previous studies on ischemic stroke.
The main limitation of our study is that it was not a randomized controlled trial, and therefore, potential biases could not be eliminated completely. It was difficult to perform this study in a randomized controlled trial setting, and therefore, cross-sectional or case-control studies could be useful for establishing the hypothesis for this topic. Second, there were no data on the previous eradication of H. pylori infection in patients, and therefore, there was a possibility that patients without current H. pylori infection could have had H. pylori infection in the past, which could affect our results. To our knowledge, no previous study has evaluated the H. pylori eradication rate in Korean patients, but we assumed that this rate might not be significantly high enough to affect our results, since the National Health Insurance covers H. pylori eradication only in patients with active peptic ulcer disease, gastric cancer, or lymphomas. The ORs for small vessel disease in H. pylori-infected patients with or without atrophic gastritis were reported to confirm our negative results, as H. pylori infection is an established risk factor for atrophic gastritis. Furthermore, although the total number of patients in this study was large, the number of patients with small vessel disease was small (n=138); therefore, it was difficult to identify trends in the location and incidence of small vessel disease in patients. Moreover, the non-significant results in this study might be attributed to inadequate statistical power. Finally, our patients were not representative of the general population because participants who undergo health-screening programs at Seoul National University Hospital are usually healthy asymptomatic adults who are more interested in their health and seek medical services more often than the nonparticipants.
In our study, no association was found between H. pylori infection and small vessel disease. Further large prospective casecontrol studies are required to prove the relationship between H. pylori infection and small vessel disease.
References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689-701.


2. Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med 2014;12:119PMID: 25012298.



3. Ochi N, Tabara Y, Igase M, Nagai T, Kido T, Miki T, et al. Silent cerebral microbleeds associated with arterial stiffness in an apparently healthy subject. Hypertens Res 2009;32:255-260. PMID: 19262493.


4. Lee SH, Park JM, Kwon SJ, Kim H, Kim YH, Roh JK, et al. Left ventricular hypertrophy is associated with cerebral microbleeds in hypertensive patients. Neurology 2004;63:16-21.


5. Ford AC, Axon AT. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 2010;15(Suppl 1):1-6.

6. Matysiak-Budnik T, Megraud F. Epidemiology of Helicobacter pylori infection with special reference to professional risk. J Physiol Pharmacol 1997;48(Suppl 4):3-17. PMID: 9440051.
7. Yim JY, Kim N, Choi SH, Kim YS, Cho KR, Kim SS, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter 2007;12:333-340. PMID: 17669107.


8. Morgando A, Sanseverino P, Perotto C, Molino F, Gai V, Ponzetto A. Helicobacter pylori seropositivity in myocardial infarction. Lancet 1995;345:1380PMID: 7752798.

9. Patel P, Mendall MA, Carrington D, Strachan DP, Leatham E, Molineaux N, et al. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ 1995;311:711-714. PMID: 7549683.



10. Whincup PH, Mendall MA, Perry IJ, Strachan DP, Walker M. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart 1996;75:568-572. PMID: 8697158.



11. Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, et al. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J 1994;71:437-439. PMID: 8011406.



12. Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet 1997;350:430-436. PMID: 9259669.


13. Heuschmann PU, Neureiter D, Gesslein M, Craiovan B, Maass M, Faller G, et al. Association between infection with Helicobacter pylori and Chlamydia pneumoniae and risk of ischemic stroke subtypes: results from a population-based case-control study. Stroke 2001;32:2253-2258. PMID: 11588309.


14. Huang WS, Tseng CH, Lin CL, Tsai CH, Kao CH. Helicobacter pylori infection increases subsequent ischemic stroke risk: a nationwide population-based retrospective cohort study. QJM 2014;107:969-975. PMID: 24890556.


15. Markus HS, Mendall MA. Helicobacter pylori infection: a risk factor for ischaemic cerebrovascular disease and carotid atheroma. J Neurol Neurosurg Psychiatry 1998;64:104-107. PMID: 9436737.



16. Ponzetto A, Marchet A, Pellicano R, Lovera N, Chianale G, Nobili M, et al. Association of Helicobacter pylori infection with ischemic stroke of non-cardiac origin: the BAT.MA.N. project study. Hepatogastroenterology 2002;49:631-634. PMID: 12063957.

17. Sawayama Y, Ariyama I, Hamada M, Otaguro S, Machi T, Taira Y, et al. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT). Atherosclerosis 2005;178:303-309. PMID: 15694938.


18. Yang X, Gao Y, Zhao X, Tang Y, Su Y. Chronic Helicobacter pylori infection and ischemic stroke subtypes. Neurol Res 2011;33:467-472. PMID: 21669114.


19. Yu M, Zhang Y, Yang Z, Ding J, Xie C, Lu N. Association between Helicobacter pylori infection and stroke: a meta-analysis of prospective observational studies. J Stroke Cerebrovasc Dis 2014;23:2233-2239. PMID: 25263434.


20. American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care 2013;36(Suppl 1):S11-S66. PMID: 23264422.


21. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611-619. PMID: 17582361.


22. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165-174. PMID: 19161908.



23. Ringelstein EB, Nabavi DG. Cerebral small vessel diseases: cerebral microangiopathies. Curr Opin Neurol 2005;18:179-188. PMID: 15791150.


Table┬Ā1
Baseline characteristics of the study population
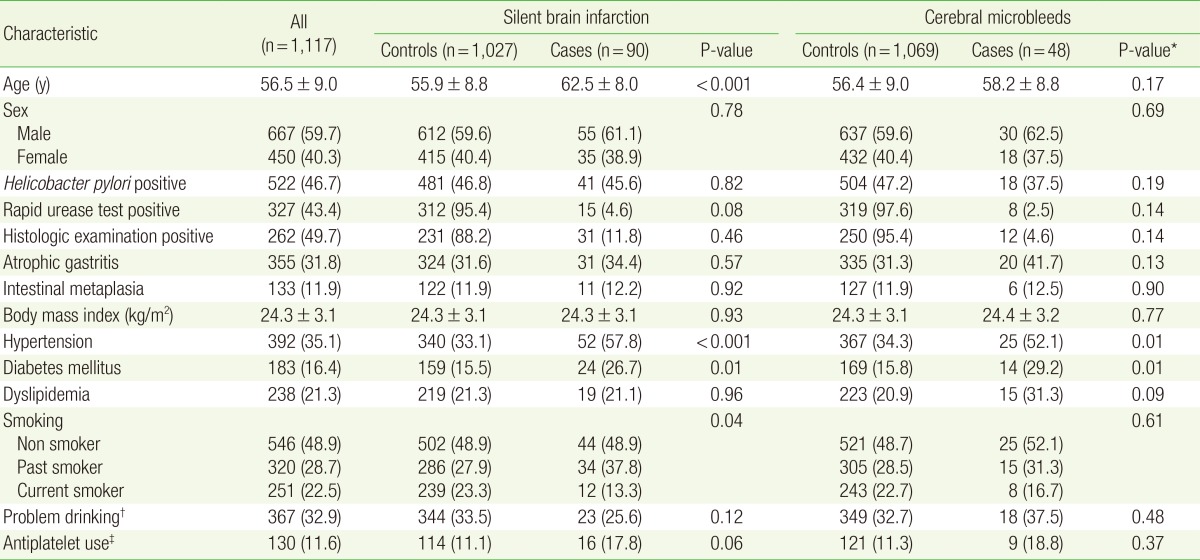
Values are presented as mean┬▒standard deviation or number (%).
*By t-test for continuous variables, by chi-square test for categorical variables. ŌĆĀProblem drinking was defined as a total alcohol intake of Ōēź40 g/wk for men and Ōēź20 g/wk for women. ŌĆĪAntiplatelet use includes the current use of aspirin or clopidogrel.
- TOOLS