|
 |
- Search
| Korean J Fam Med > Volume 33(5); 2012 > Article |
Abstract
Background
As many studies revealed that oxidative stress due to the imbalance of reactive oxygen species (ROS) and antioxidant capacity is related with pathologic processes such as cardiovascular diseases, diabetes, as well as aging and obesity, the relationship between lifestyle and oxidative stress has recently gained much medical attention. However, little information exists on the effects of lifestyle on ROS in Korea. In this study, we investigated the effects of lifestyle on free oxygen radical levels in men and women in Korea.
Methods
A total of 138 adults participated in this study from September 2007 to June 2010 at a health promotion center and department of family medicine. Information on the lifestyle of each participant was obtained by questionnaire. Biochemical markers and a free oxygen radical test (FORT) were also measured.
Results
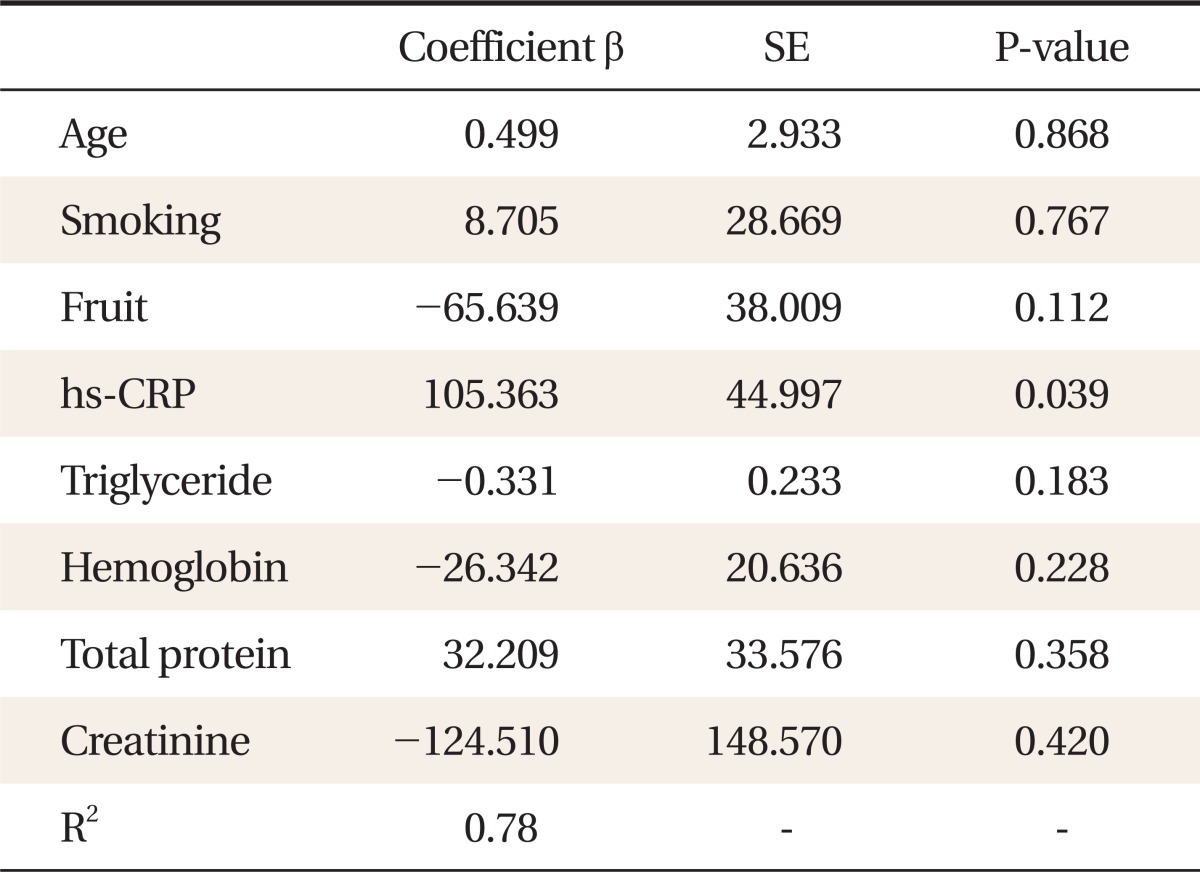
The average age was 47.28 ± 10.85 years and 79.7% were male. High sensitivity C-reactive protein (hs-CRP; r = 0.418, P = 0.012), triglycerides (r = -0.243, P = 0.008), hemoglobin (r = -0.445, P < 0.001), total protein (r = 0.210, P = 0.036), creatinine (r = -0.294, P = 0.001), fruit intake per day (P = 0.047), and smoking (P = 0.003) were related to the FORT levels in univariate analysis. Multiple linear regression analysis showed that hs-CRP (P = 0.039) was an independent predictor of serum FORT values. This statistical model can explain 78% of the variance in FORT values.
Reactive oxygen species are formed as a result of the metabolism of oxygen within cells for energy generation in the process of respiration. Excessively formed free radicals damage the cell membrane, DNA, and other cellular structures by inducing oxidation reactions. Furthermore, depending on the scope of damage, the cell may loose its ability to function or function may be altered in cells. Normally, the level of reactive oxygen is maintained in balance with that of antioxidants, but when an imbalance occurs in the form of surplus of reactive oxygen or shortage of antioxidants, this is referred as oxidative stress. Oxidative stress is significantly associated with arteriosclerosis, cancer, cardiovascular diseases, diabetes, inflammatory diseases, obesity, and aging.1-5)
Meanwhile, various lifestyle patterns, nutritional factors, environmental factors, and genetic factors are reported to be associated with occurrence of oxidative stress due to excess free radicals and reduced antioxidant capacity.6) According to studies of antioxidant capacity conducted on healthy adults as subjects, males and the elderly had a negative correlation with antioxidant capacity. Abstinence from smoking, intake of vitamins and minerals, routine exercise, and fruit intake were reported to have a positive correlation with overall antioxidant capacity. Moreover, mental stress, alcohol consumption, and excessive exposure to the ultraviolet (UV) rays were reported to be associated with reduction of antioxidant capacity.7)
Many studies assessed oxidative stress using the free oxygen radical test (FORT) values in patients with type 2 diabetes, cardiovascular diseases such as myocardial infarction, cancer, and others, and presented research results on their relationships.8-12)
However, domestic studies on the effects of various lifestyle patterns on reactive oxygen species (ROS) have been inadequately conducted and study subjects were limited to certain populations. In addition, a number of studies used methods other than the FORT for assessment of oxidative stress. Therefore, this study examined the effects of lifestyle, eating habits, disease, and other factors on the FORT values in the Korean adult population.
This study was implemented in accordance with ethical and safety guidelines upon the approval of the institutional review board in The Catholic University of Korea St. Vincent's Hospital (VC11 RISI0102).
A total of 138 adults participated in this study from September 2007 to June 2010 at a health promotion center and department of family medicine located in Gyeonggi province. Among 141 consenting participants, those who responded to questionnaires with insufficient answers or missing FORT evaluations were excluded from the sample.
The research used self-report questionnaires to assess lifestyle, eating habits, and disease history of participants. Sufficient explanation of the purpose of the study and proper guidelines for answering the questionnaire were provided a priori. The questionnaires were self-administered and data collected with the consent of participants. The survey included items on alcohol history, smoking history, amount of exercise, living environment, sun exposure, vitamin intake, eating habits, diabetes, high blood pressure, cardiovascular disease, apoplexy history, allergic diseases (i.e., asthma, allergic rhinitis, allergic dermatitis, and atopic dermatitis), number of times contracting a cold per year, and others. We conducted the analysis dividing between endogenous and exogenous factors. Endogenous factors include age, gender, amount of exercise, allergic diseases, history of diabetes, high blood pressure, cardiovascular diseases, and apoplexy. Exogenous factors include smoking history, alcohol history, living environment, sun exposure, vitamin intake, eating habits, and number of times contracting a cold per year. Regarding the amount of exercise, groups were divided into a regularly exercising group with one or more times a week, an irregularly exercising group with less than once a week, and a group exercising not at all. Regarding eating habits, the number of fruit and vegetable servings consumed each day, and grilled meat and fast food intake per week were taken into consideration. Meanwhile, history of diabetes, high blood pressure, cardiovascular diseases, and apoplexy were divided into cases without any diseases, cases with disease, and cases with family history but without any diseases currently.13) Moreover, the Korean version of Brief Encounter Psychosocial Instrument (BEPSI) was used for evaluation of the study population's stress level. The BEPSI was developed by Yim et al.14) and its reliability is confirmed with Cronbach's alpha of 0.71. The survey asks patients to report their own degree of stress by choosing from answers ranging from degree 1, "always" to degree 5, "never" in all of the five questions. According to the average score of the stress measure, patients were later categorized into 3 groups: mild, moderate, and severe.
After more than 8 hours of fasting, blood samples were collected to examine the total amount of cholesterol, triglycerides, high-density lipoproteins, low-density lipoproteins, albumin, total protein, uric acid, high sensitivity C-reactive protein (hs-CRP), glycated hemoglobin, aspartate transaminase, alanine transaminase, gamma-glutamyl transpeptidase, blood urea nitrogen, creatinine, lipoprotein A, thyroid-stimulating hormone, the total number of white blood cells, and blood pigments.
The FORT was evaluated by assessing hydrogen peroxide (H2O2) levels within the blood using the blood samples obtained from peripheral blood vessels of the tip of a finger using the FORT test (Callegari, Parma, Italy) reagent. This test is based on the principle that when Alkoxyl radicals and peroxyl radicals formed during oxidation and de-oxidation of peroxide and hydrogen peroxide by Fe2+ (ferrous iron) and Fe3+ (ferric iron), are combined with CrNH2, develop differing degrees of colors. Thus, by using the color comparison method, one can compare and measure the concentration of free oxygen radicals. The free radical analysis system (free oxygen radicals monitor [FORM] Plus; Callegari) was used in the assessment. Analysis results were presented with FORT units and 1 FORT unit is equal to 0.26 mg/L H2O2. Although only the measure of hydrogen peroxide is observable using FORM, because the FORT reagent is packed in a disposable container using freeze dried storage, it is simpler to compare to conventional ROS test methods. Moreover, it also shortens the total examination time taken, since except for blood collection procedure, all the processes, inclusive of the first and second assessment phases and the output of results, are automated. In addition, it is also possible to conduct a wide-range (160-600 FORT units) of tests with a high reproduction rate. Since the test requires a small volume of blood sample (20 µL), it reduces the fears that could be felt by patients and thus can be conveniently performed. Since reliability, validity, and reproduction of FORT are proven, the test has been widely used in the analysis of free radicals of oxygen produced in the body.8,10,11,15-21) In particular, the study by Palmieri and Sblendorio2,17) showed that intra-assay coefficient of variation and inter-assay coefficient of variation of FORT were 3.7% and 6.2%, respectively.
Collected data were analyzed by using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance and t-test were performed to examine the relationships of ROS with lifestyle, eating habits, and the presence of disease. Pearson's correlation analysis was performed to identify the relationship biochemical markers and free oxygen radical levels. Finally, multiple linear regression analysis was performed to analyze the effects of various independent variables on the FORT. P-values less than 0.05 were regarded as statistically significant.
A total of 138 subjects participated in the study and their average age was 47.28 ± 10.85 years. The subjects comprised 110 males (79.7%) and 28 females (20.3%). There was no significant difference when the FORT values were compared over age, amount of exercise, presence of allergic diseases, and history of diabetes, high blood pressure, cardiovascular disease, and apoplexy. Moreover, no significant correlation was found when the FORT values were compared depending on the degree of stress using BEPSI-Korean, the Korean version (Table 1).
The FORT values in present smoking group, past smoking group, and non-smoking group were 271.49 ± 53.64 FORT units, 296.48 ± 78.85 FORT units, and 326.78 ± 79.59 FORT units, respectively, depending on the smoking habits, showing a statistically significant high value in the non-smoking group (P = 0.003). The FORT values were 306.67 ± 76.08 FORT units in the group eating fruit no more than once a day; and 278.41 ± 68.25 FORT units in the group eating fruit more than twice a day depending on the fruit intake per day. A statistically significant high FORT value was shown in the group eating fruit no more than once a day (P = 0.047). Furthermore, no significance was found when the FORT values were compared with other exogenous factors including alcohol consumption per week, living environment, sun exposure, vitamin intake, the vegetable intake per day, grilled meant and fast food intake per week, and number of times contracting a cold per year (Table 2).
The FORT values exhibited negative correlations with hemoglobin (P < 0.001), creatinine (P = 0.001), and triglycerides (P = 0.008), according to analysis on the relationships between biochemical markers and the FORT values. In contrast, positive correlations were shown with total protein (P = 0.036) and hs-CRP (P = 0.012). There was no statistically significant relationship between the FORT values and other biochemical markers (Table 3). Profound positive relationships were shown between hs-CRP and the FORT values in a scatter diagram (Figure 1).
Multiple linear regression analysis was conducted to assess the effects of significant variables on the FORT values in univariate analysis including age. The analysis showed that hs-CRP was an independent predictor of FORT values. R2-value was 0.78 (Table 4).
This study examined the effects of FORT values on various lifestyles, eating habits, the presence of disease, and other factors. First, no significant difference was present when the FORT values were compared with endogenous factors such as age, amount of exercise, degree of stress, the presence of allergic diseases, or others. In addition, no significant difference was shown when the FORT values were compared with exogenous factors such as weekly alcohol consumption, living environment, sun exposure, vitamin intake, daily number of servings of vegetables, weekly grilled meat and fast food consumption, and number of times contracting a cold per year. Moller et al.6) reported that alcohol consumption, smoking, emotional stress, exposure to UV rays, air pollution, and other factors may cause an increase in oxidative stress. Lesgards et al.7) also found that antioxidant capacity had a negative correlation with increasing age in the case of males in the study on the effects of various lifestyles of adults on antioxidant capacity. The study also showed that antioxidant capacity had a positive correlation with intake of vitamins and minerals, and routine exercise. When the FORT values were compared depending on smoking habits, the values were statistically significantly high (P = 0.003) in the non-smoking group. Unlike earlier studies,22,23) an inverse correlation was found between smoking and ROS. However, smoking had no relationship to free radical values in the analysis of effects of various independent variables on the FORT values. Lorgis et al.9) presented similar results in their study, reporting that no significant difference was found when the FORT values were compared depending on smoking habits. However, there are many studies showing diverse results on the effects of smoking on ROS including the study of Lesgards et al.7) which exhibited a positive correlation between non-smoking and antioxidant capacity. Since tobacco smoke contains massive amounts of ROS and oxidizing agents besides from nicotine, the mechanism of atherosclerosis is oxidative deformation of low-density lipoprotein cholesterol within the plasma due to free oxygen radicals and other products formed during smoking.24) However, as stated above, study results on the relationship between smoking and oxidative stress are inconsistent. Therefore, additional studies are needed to test the relationship under controlled environments.
When the FORT values were compared depending on fruit intake per day, the FORT value was statistically and significantly high (P = 0.047) in the group eating fruit no more than once a day. Since the study was not conducted on the relationship between eating habits and ROS, direct comparison was not available. However, a positive correlation was found between antioxidant capacity and fruit intake in the study of Lesgards et al.7) Furthermore, a study by Cao et al.25) showed similar results to this study, that is, an increase in fruit and vegetable intake was effective in boosting blood antioxidant capacity. Fruit intake was shown to have statistically significant correlation with the FORT values in the analysis of the effects of various independent variables on the FORT values. Although a declining tendency in the FORT values was observed as fruit intake increased, such results are assumed to be similar to those of previous studies. Additional studies with a greater sample population should be performed in the future.
The FORT values exhibited negative correlations with hemoglobin (P < 0.001), creatinine (P = 0.001), and triglycerides (P = 0.008) in terms of biochemical markers. On the other hand, they showed positive correlations with total protein (P = 0.036) and high sensitivity C-reactive protein (P = 0.012). However, only hs-CRP was actually related to the FORT value in the analysis of the effects of various independent variables on the FORT values. The result is thought to be practically due to influences of various other variables. hs-CRP is an inflammatory marker which has been assessed in numerous studies and has been used especially as a potential marker of the risk of cardiovascular diseases.19,26) Lorgis et al.9) conducted a study on 66 patients with acute myocardial infarction to investigate the relationships among the FORT values as the evaluation tool of oxidative stress, and clinical and biochemical markers. According to multiple linear regression analysis, the study reported that the independent predictable factor of FORT values were, in case of hs-CRP, (P = 0.023), and left ventricular ejection fraction <40% (P < 0.001), and in case of diabetes (P = 0.039). Abramson et al.10) reported that a profound positive correlation was shown between the FORT values and hs-CRP in a study conducted on 126 adults without any coronary heart disease to identify the relationship between hs-CRP and oxidative stress marker. In addition, Garelnabi et al.18) also identified a positive correlation between the FORT values and hs-CRP. In this study, utilization of hs-CRP as an oxidative stress predictable factor could be taken into consideration since a profound positive correlation was exhibited between the FORT values and hs-CRP. There was no significant difference shown when the FORT values were compared depending on the history of diabetes, high blood pressure, cardiovascular disease, and apoplexy. However, the FORT values were high in type 2 diabetes compare to the control group, and free oxygen radicals defence was shown to be low in the study of Pavlatou et al.8) ROS and antioxidant capacity are crucial in the occurrence and assessment of oxidative stress. Moreover, the imbalance of ROS and antioxidant capacity could induce oxidative damage and it is shown to be profoundly interrelated to the mechanism of various diseases including type 2 diabetes and others.
This study has some limitations. First, although the study was able to assess the relationships between various lifestyle patterns with the FORT values, this study was not able to identify a precise causal relationship. Since the study was conducted as a sectional investigation, chronological relationships among variables were unable to be identified. Second, since the study population was composed of 110 males (79.7%) and 28 females (20.3%), accurate data comparison between genders was difficult due to a relatively higher number of male subjects. In addition, the total number of subjects was insufficient. Third, since contractions of diseases were investigated based on the self administrated questionnaires, the actual diagnosis and treatments of diseases could be different. Fourth, although the FORT values could vary according to the influences of various eating habits and genetic and environmental factors, this study solely investigated the relationship between the FORT values and certain eating habits and environmental factors. Fifth, the BEPSI, the valid analysis method, was applied for the stress assessment of subjects. Although no significant difference was shown in the FORT values over the degrees of stress measured using the BEPSI, additional studies are necessary to further investigate whether or not FORT values reflect stress levels and are actually associated with the degree of stress. Finally, this study measured the FORT values by assessing hydrogen peroxide levels in the blood. Since the research did not evaluate antioxidant capacity, there is a limitation in assessing accurate overall oxidative stress levels.
Expanding on the findings of previous studies, since there is a possibility of higher oxidative stress in the case of people with high levels of hs-CRP, this study is significant in providing evidence for the necessity and importance of ROS examination. Moreover, sufficient fruit intake is thought to be helpful in reducing free oxygen radicals. Additional large-scale studies are thought to be essential to further investigate the relationship between ROS and lifestyle, eating habits, and presence of disease. Furthermore, oxidative stress needs to be assessed more accurately by conducting studies on oxidation capacity at the same time, as well as free oxygen radicals.
References
1. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84. PMID: 16978905.


2. Palmieri B, Sblendorio V. Oxidative stress tests: overview on reliability and use. Part I. Eur Rev Med Pharmacol Sci 2007;11:309-342. PMID: 18074940.

3. Steinberg D. Antioxidants in the prevention of human atherosclerosis: summary of the proceedings of a National Heart, Lung, and Blood Institute Workshop: September 5-6, 1991, Bethesda, Maryland. Circulation 1992;85:2337-2344. PMID: 1591855.


4. Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001;54:176-186. PMID: 11253127.



6. Moller P, Wallin H, Knudsen LE. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem Biol Interact 1996;102:17-36. PMID: 8827060.


7. Lesgards JF, Durand P, Lassarre M, Stocker P, Lesgards G, Lanteaume A, et al. Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects. Environ Health Perspect 2002;110:479-486. PMID: 12003751.



8. Pavlatou MG, Papastamataki M, Apostolakou F, Papassotiriou I, Tentolouris N. FORT and FORD: two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009;58:1657-1662. PMID: 19604518.


9. Lorgis L, Zeller M, Dentan G, Sicard P, Richard C, Buffet P, et al. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis 2010;213:616-621. PMID: 20947086.


10. Abramson JL, Hooper WC, Jones DP, Ashfaq S, Rhodes SD, Weintraub WS, et al. Association between novel oxidative stress markers and C-reactive protein among adults without clinical coronary heart disease. Atherosclerosis 2005;178:115-121. PMID: 15585208.


11. Mantovani G, Madeddu C, Maccio A, Gramignano G, Lusso MR, Massa E, et al. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol Biomarkers Prev 2004;13:1651-1659. PMID: 15466983.



12. Gizi A, Papassotiriou I, Apostolakou F, Lazaropoulou C, Papastamataki M, Kanavaki I, et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol Dis 2011;46:220-225. PMID: 21334230.


13. Challem J, Block M. Basic Health Publications user's guide to antioxidant supplements: discover how natural antioxidants can reduce your risk of heart disease, cancer, and alzheimer's disease. 2005. North Bergen: Basic Health Publications.
14. Yim JH, Bae JM, Choi SS, Kim SW, Hwang HS, Huh BY. The validity of modified Korean-translated BEPSI (brief encounter psychosocial instrument) as instrument of stress measurement in outpatient clinic. J Korean Acad Fam Med 1996;17:42-53.
15. Curello S, Ceconi C, Cargnoni A, Cornacchiari A, Ferrari R, Albertini A. Improved procedure for determining glutathione in plasma as an index of myocardial oxidative stress. Clin Chem 1987;33:1448-1449. PMID: 3608163.


16. Griffiths HR, Moller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, et al. Biomarkers. Mol Aspects Med 2002;23:101-208. PMID: 12079771.


17. Palmieri B, Sblendorio V. Oxidative stress tests: overview on reliability and use. Part II. Eur Rev Med Pharmacol Sci 2007;11:383-399. PMID: 18306907.

18. Garelnabi MO, Brown WV, Le NA. Evaluation of a novel colorimetric assay for free oxygen radicals as marker of oxidative stress. Clin Biochem 2008;41:1250-1254. PMID: 18703037.


19. Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004;109(25 Suppl 1):IV6-IV19. PMID: 15226246.


20. Harris MT, Davis WW, Le NA, Eggleston B, Austin GE, Moussa M, et al. Free oxygen radicals in whole blood correlate strongly with high-sensitivity C-reactive protein. J Clin Lipidol 2007;1:593-598. PMID: 21291700.


21. Kim DH, Kang HS. Effect of alkaline ionized water supplementation on anaerobic capacity and scavenges active oxygen species. J Sport Leis Stud 2008;34:1157-1169.

22. Campos C, Guzman R, Lopez-Fernandez E, Casado A. Urinary biomarkers of oxidative/nitrosative stress in healthy smokers. Inhal Toxicol 2011;23:148-156. PMID: 21391783.


23. Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur J Obstet Gynecol Reprod Biol 2011;155:132-136. PMID: 21216085.


24. Yamaguchi Y, Matsuno S, Kagota S, Haginaka J, Kunitomo M. Oxidants in cigarette smoke extract modify low-density lipoprotein in the plasma and facilitate atherogenesis in the aorta of Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2001;156:109-117. PMID: 11369003.


25. Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr 1998;68:1081-1087. PMID: 9808226.


26. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363-369. PMID: 12551853.


Figure 1
Analysis of the correlation between free oxygen radical test (FORT) values and high sensitivity C-reactive protein (hs-CRP).
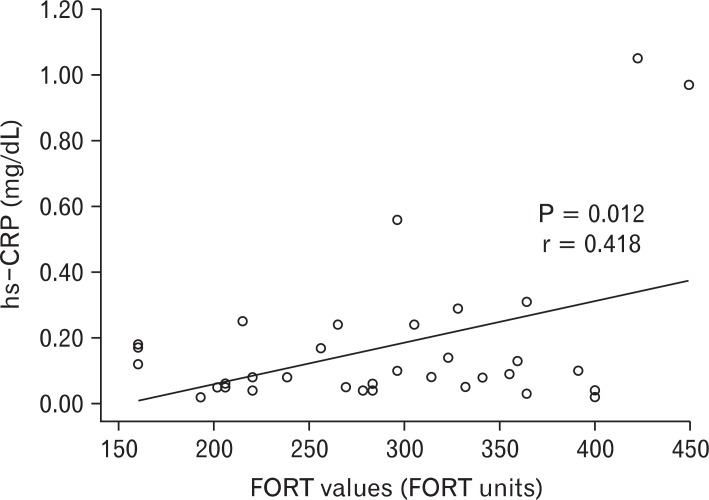
Table 1
Relationship between endogenous factors of the study population and levels of reactive oxygen species measured by the free oxygen radicals test (FORT).
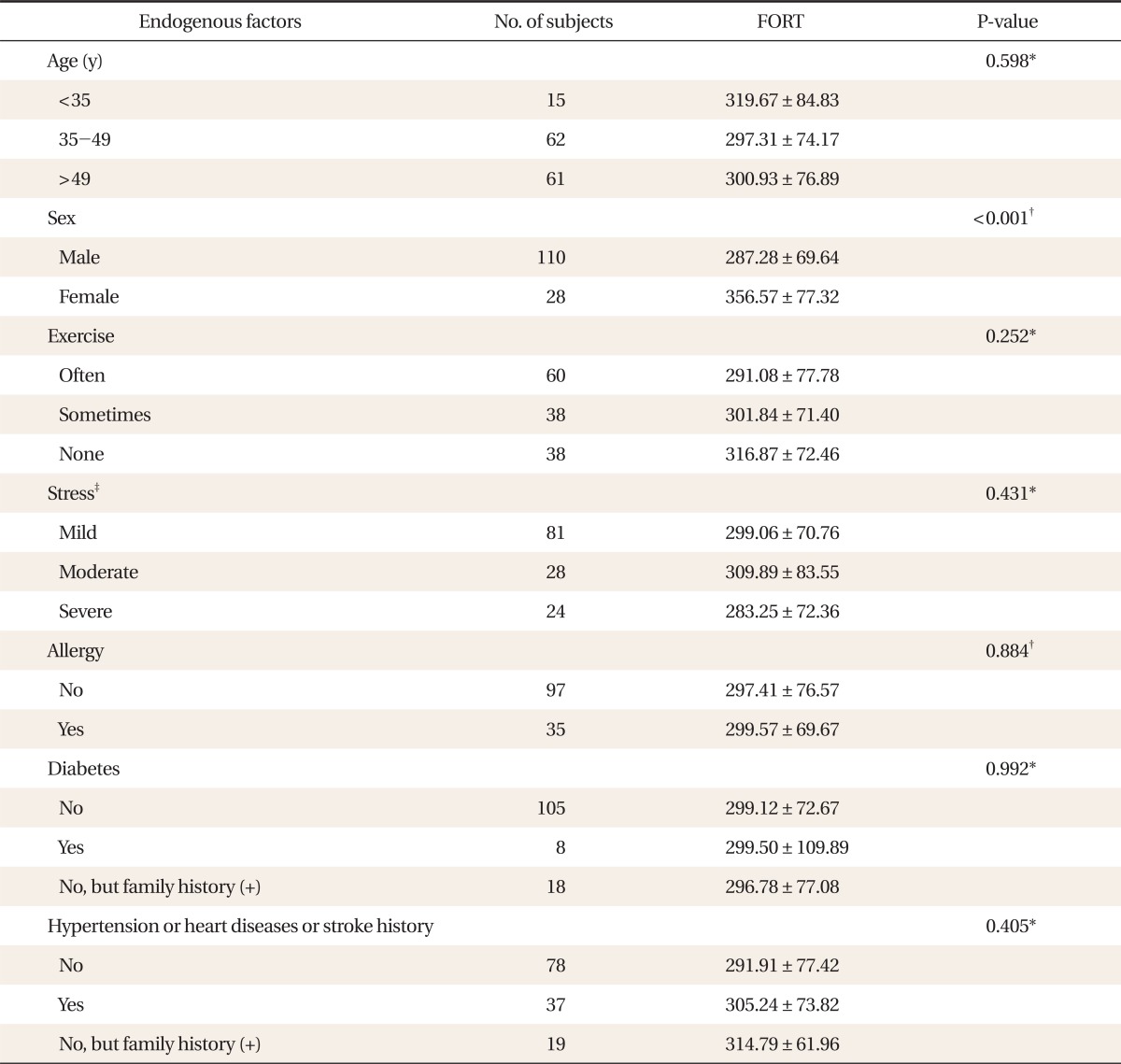
Table 2
Relationship between exogenous factors of the study population and levels of reactive oxygen species measured by the free oxygen radical test (FORT).
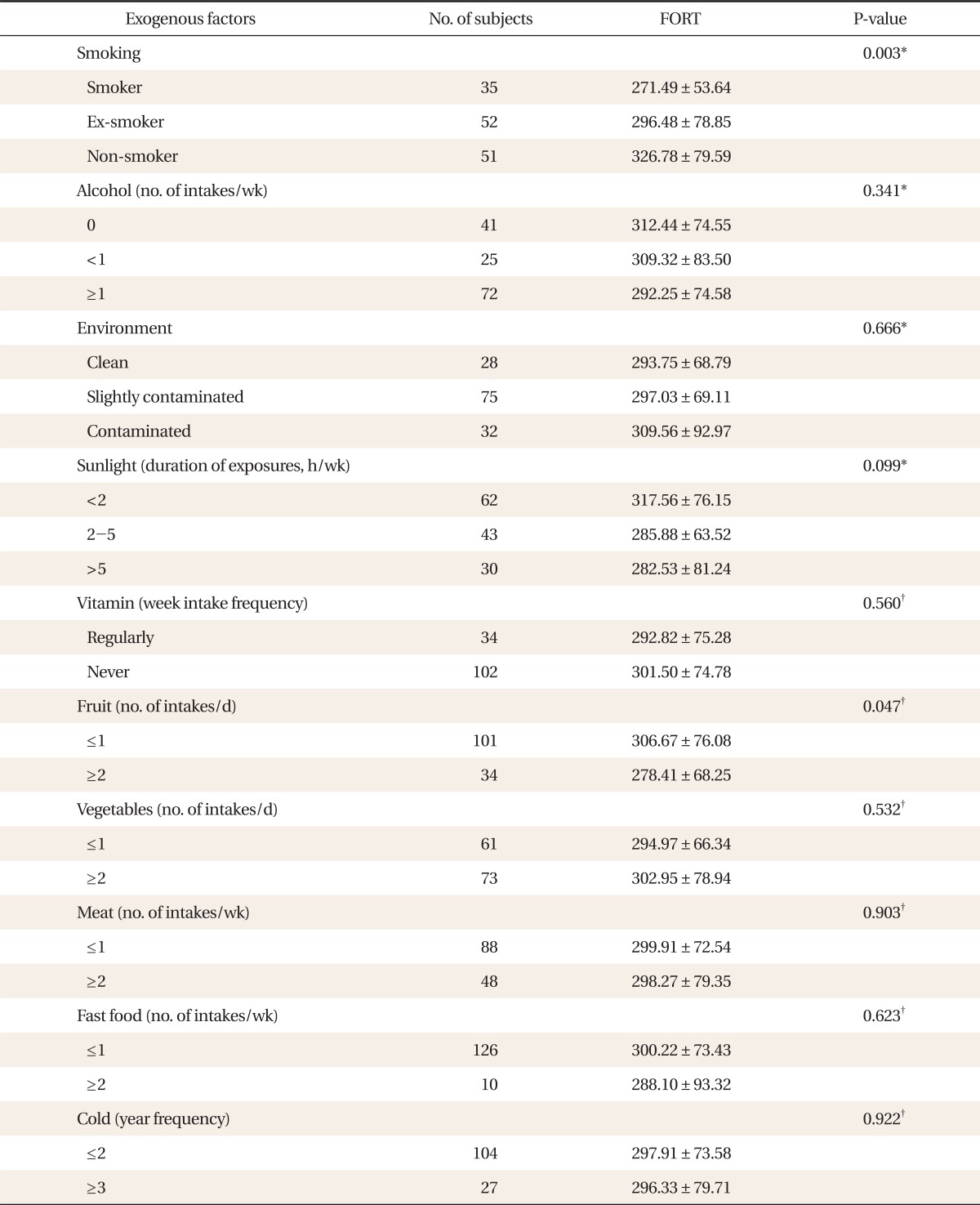
Table 3
Relationship between biological data of the study population and reactive oxygen species levels measured by the free oxygen radical test.
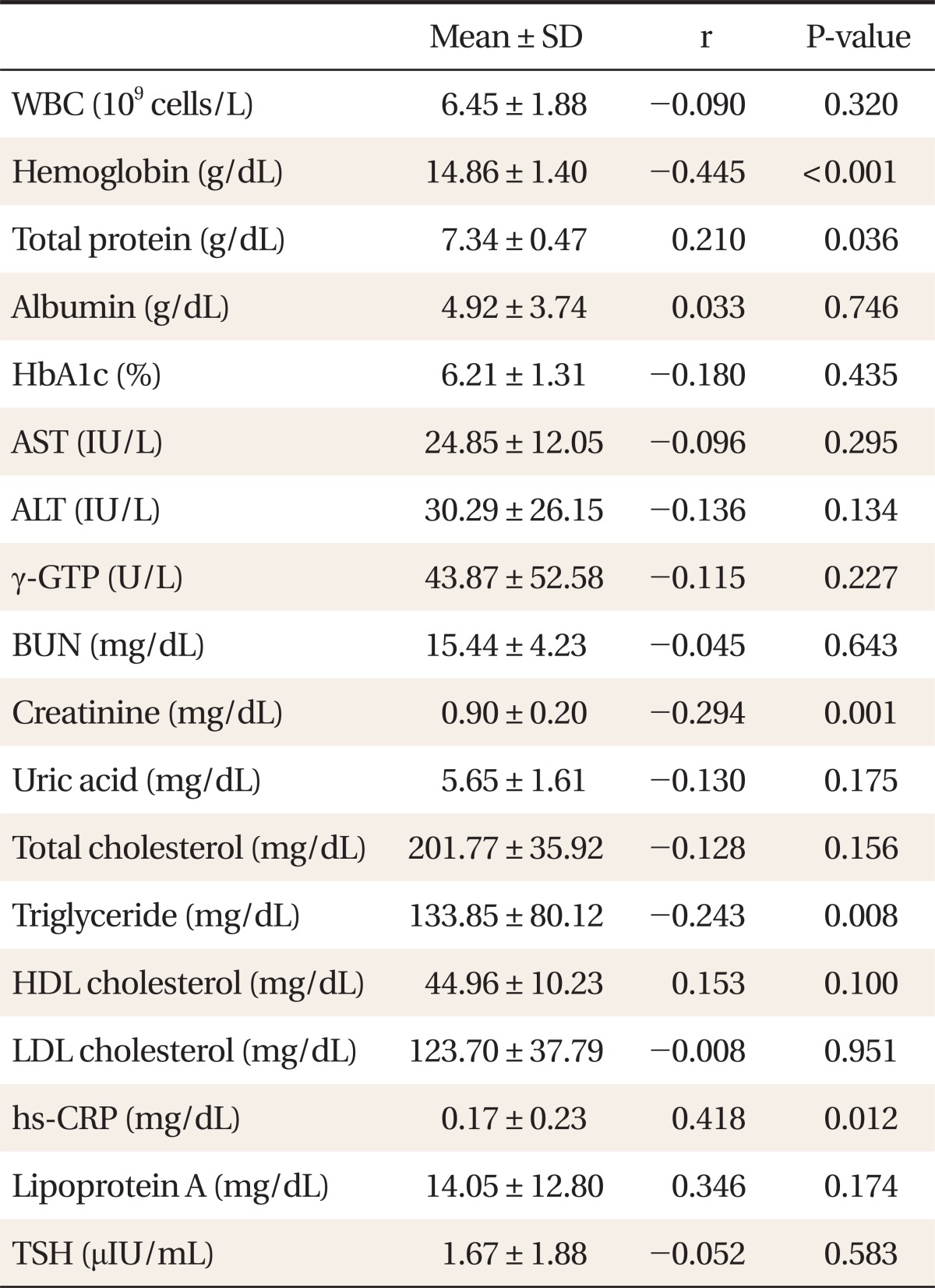
P-values were obtained by Pearson's correlation analysis.
WBC: white blood cell count, HbA1c: hemoglobin A1c, AST: aspartate transaminase, ALT: alanine transaminase, γ-GTP: gamma-glutamyl transpeptidase, BUN: blood urea nitrogen, HDL: high-density lipoprotein, LDL: low-density lipoprotein, hs-CRP: high sensitivity C-reactive protein, TSH: thyroid-stimulating hormone.