Correlation between Frailty and Cognitive Function in Non-Demented Community Dwelling Older Koreans
Article information
Abstract
Background
Frailty and cognitive impairment are considered the most common and yet least understood conditions in older adults. This study was conducted to investigate the correlation between frailty and cognitive function in non-demented older Koreans.
Methods
Korean Mini-Mental Status Examination (K-MMSE) scores and Cardiovascular Health Study Frailty Indices were obtained for 486 older adults aged 65 and over who registered at six senior welfare centers in Seoul and Gyeonggi province. Multiple linear regression was performed to identify the association between frailty and K-MMSE scores.
Results
Of the 486 older adults, 206 (42.4%) were robust, 244 (50.2%) were prefrail, and 36 (7.4%) were frail. Prevalence of cognitive impairment (K-MMSE ≤ 23) was 6.3% in the robust group, 16.8% in the prefrail group, and 30.6% in the frail group (P < 0.001), and mean K-MMSE score was 27.5 ± 2.2, 26.5 ± 3.1, and 23.7 ± 5.3, respectively (P < 0.001). Frailty tended to be associated with lower MMSE scores (B = -1.92, standard error, 0.52; P < 0.001).
Conclusion
Frailty was found to be correlated with cognitive impairment in non-demented older Koreans. However, further cohort studies are required to determine the association between frailty and cognitive function.
INTRODUCTION
South Korea faces a challenge posed by a rapidly growing older population (>65 years old), which increased from 7.2% of the total population in 2000 to 11% in 2010. Furthermore, this growth is expected to continue to 14.3% in 2018 and 20.8% in 2026.1,2) Increases in older populations can cause economic and medical burdens associated with taking care of dependent older adults with physical or mental impairments,3) and frailty and cognitive impairment are considered as the most common and yet least understood conditions in older adults3))
Frailty is an age-related reduction in the ability to respond to stressors and increased vulnerability to weakness, morbidity, disability, and death.4) No consensus has been reached regarding the definition of frailty but two types of operational definitions have been commonly used.3) Rockwood et al.5) proposed the Cumulative Burden Index that frailty that be defined as an accumulation of health conditions and deficits. Cognitive impairment itself can be included as one of possible deficits. Fried et al.4) proposed the Biological Syndrome Model that be defined as a condition that satisfies at least three of five objective criteria, that is weight loss, exhaustion, reduced physical activity, reduced gait speed, and weakness. Fried et al.4) did not include cognitive function in its definition because they suggested that physical frailty was one of causes resulting in adverse outcome as cognitive impairment.
Cognitive impairment describes a decline in intellectual functions, such as, thinking, remembering, reasoning and planning,3)) and ranges from mild forms of forgetfulness to severe and debilitating dementia. Mild cognitive impairment is a state of cognitive decline that is not accompanied by any significant functional disability,6) but shows a high rate of progression to all types of dementia in which severe cognitive impairment is accompanied by increasing physical decline, eventually leading to full physical dependency.3)
Several studies provided evidence of a relationship between frailty and cognitive impairment. Avila-Funes et al.7) reported higher rates of cognitive impairment in frail (22%) than in pre-frail (12%) or robust (10%) older people, and many longitudinal studies have suggested that higher levels of frailty predict cognitive decline8,9,10) and incident dementia.11,12) Samper-Ternent et al.10) reported that subjects in the frail category showed faster and more severe cognitive decline over 10 years than those in the prefrail category. On the other hand, some studies concluded that cognition was not correlated strongly with frailty, while they define frailty as one concept consisting of physical activity, mobility, energy, strength and mood.13,14)
If frailty is shown to cause cognitive impairment, interventions to reduce frailty may prevent cognitive decline. Although some studies have been conducted on frailty in older Koreans, the relationship between frailty and cognitive function in older Koreans has received little attention. Hence, we aimed to investigate the correlation between frailty and cognitive function in non-demented community dwelling older Koreans by using data collected during two studies the Validity and Reliability of Korean Frailty Index15) and the Validity and Reliability of the Kaigo-Yobo Checklist in Korean Elderly.16)
METHODS
1. Participants
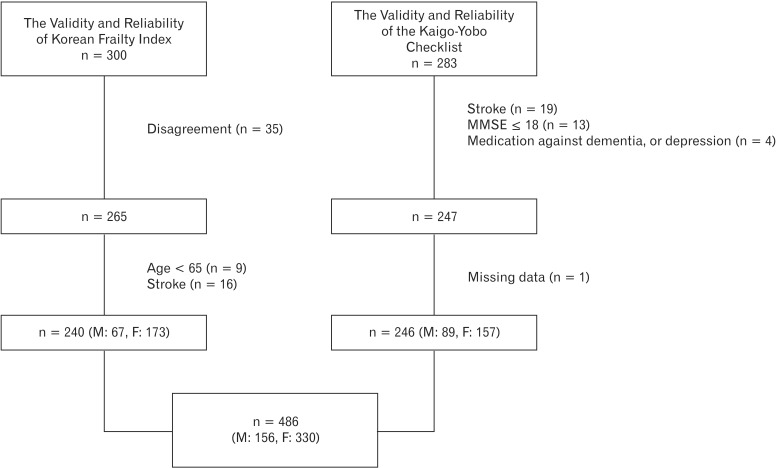
Study population was composed of individuals that participated in the Validity and Reliability of Korean Frailty Index15) and the Validity and Reliability of the Kaigo-Yobo Checklist in Korean Elderly (Figure 1).16)
In the Validity and Reliability of Korean Frailty Index,15) initially 300 older adults (≥65 years old), registered at three senior welfare centers located in Seoul were randomly selected. After explaining the purpose of this study, 265 elderly (Sungdong welfare center, n = 53; Sillim welfare center, n = 138; Jung-gu Yoo-Rock welfare center, n = 74), who understood the purposes of the study and provided signed consent, were included. A structured survey and physical examination were performed on these candidate subjects from June 2009 to August 2009. Participants in the survey were limited to those who could understand the survey contents and answer questions adequately. Those unable to stand independently or walk more than 10 meters without assistance due to symptoms that restrict motility, such as, dizziness, a visual disorder, or shortness of breath, were excluded. However, we included individuals who had difficulty reading the questionnaire due to a vision problem or a lack of education by reading the questions to them and filling the questionnaires on their behalf. Patients with Parkinson disease, a previous history of stroke, dementia (a Korean Mini-Mental State Examination [K-MMSE] score of < 18), and patients taking drugs for dementia or depression were excluded. Of the 265 subjects who participated in the original survey, 25 subjects with an age of < 65 (n = 9) or a history of stroke (n = 16) were also excluded. These inclusion and exclusion criteria were adopted from the Cardiovascular Health Study (CHS), as described by Ferrucci et al.17)
In the validity and reliability of the Kaigo-Yobo checklist in Korean elderly study,16) 283 older adults (≥65 years old) registered at two senior welfare centers located in Seoul (n = 37 and n = 238) and one senior welfare center in Gyeonggi province (n = 8) were recruited from March, 2011 to May, 2011. According to the exclusion criteria of Ferrucci et al.,17) 36 subjects were excluded (stroke, n = 19; K-MMSE score < 18, n = 13), and taking drugs for dementia or depression (n = 4). In addition, missing data (n = 1) was also excluded. The study protocols were approved beforehand by the Hanyang University Hospital institutional review board (HYUH IRB No. 2008-R-38 and HYUH IRB No. 2010-R-47).
2. Measures
1) Cognitive function assessment: Korean Mini-Mental State Examination
Cognitive function was assessed using the K-MMSE, the Korean version of Mini-Mental State Examination (MMSE). The MMSE was developed by Folstein in 1975 and has been widely used to measure cognitive function in clinical and research settings.18) The K-MMSE was developed to maintain the sense of the questions posed in the English MMSE as much as possible and its validity and reliability were verified by Kang et el.19) K-MMSE scores range from 0 to 30, and lower scores indicate poorer cognition. The K-MMSE awards 5 points for time orientation, 5 points for place orientation, 3 points for registration, 3 points for recall, 5 points for attention and calculation, 8 points for language, and 1 point for visual construction. Cognitive impairment was defined as a K-MMSE score of below 23 points (≤23).20)
2) The Cardiovascular Health Study Frailty Index
Fried proposed that frailty be defined as a condition meeting at least three of the following five items; weight loss, exhaustion, reduced walking speed, reduced physical activity, and reduced grip strength. Subjects with 1 to 2 positive items are classified as prefrail, and those with no positive item as robust.4)
(1) Weight loss
Weight loss was defined as unintentional weight loss of >4.5 kg (or 5% of body weight) over the previous year (score = 1).
(2) Exhaustion
Two items from the Center for Epidemiological Studies-Depression scale were used to assess exhaustion: "I feel that everything I do is an effort." and "I cannot get going." Subjects answering "yes" at least 3 days per week to either of these two items were assigned one point (score = 1).
(3) Low physical activity
Calories consumed per week were calculated using the International Physical Activity Questionnaire-Short Form, the reliability and validity of which have been demonstrated by domestic and international studies.21) The cut-off values of physical activity in this study was 462 kcal per week for males and 297 kcal per week for females, which represent the values of the lowest 20% obtained by reanalyzing data collected during the Survey on health and welfare status of the elderly in Korea 2008, which was conducted in 10,715 community-dwelling older Koreans aged ≥ 65 years.22) Subjects with values lower than the above-mentioned cut-off values were assigned one point (score = 1).
(4) Reduced gait speed
A standard walking speed was set by measuring the time taken to walk 4.5 m. Subjects were asked to walk 6 m at a comfortable walking speed. Times taken to walk 4.5 m, excluding the starting and ending portions of 0.75 m, were measured twice and the shorter time was selected. We adopted cut-off values representing values of the lowest 20% obtained by reanalyzing data collected during the survey on health and welfare status of the elderly in Korea 2008, which was conducted in 10,715 community-dwelling older Koreans aged ≥ 65 years.22) However, the Survey on health and welfare status of the elderly in Korea 2008 measured times taken to walk 2.5 m. Subjects lower than the cut-off value were assigned one point (score = 1).
(5) Reduced grip strength
Grip strength was measured twice using a dynamometer (JAMAR hydraulic hand dynamometer; Sammons Preston, Bolingbrook, IL, USA). Subjects were asked to grip the dynamometer with lowered straight arms without allowing the dynamometer to touch the body. Hand grip strengths were measured in kg twice and higher values were used in the analysis. We used the cut-off values of the lowest 20% adjusted for sex and body mass index (BMI) obtained by reanalyzing data collected during the survey on health and welfare status of the elderly in Korea 2008, which was conducted in 10,715 community-dwelling older Koreans aged ≥ 65 years using a dynamometer (TANITA Hand Grip Meter Bule 6103; Tanita Co., Tokyo, Japan).22) Subjects with grip strengths lower than the cut-off value were assigned one point (score = 1).
3) Chronic disease
Chronic diseases were assessed using self reported questio-nnaires, which including a series of questions about whether they had ever been told by a doctor that they had myocardial infarction (MI), angina, congestive heart failure (CHF), peripheral vascular disease (PVD), arthritis, cancer, diabetes, hypertension, or chronic obstructive pulmonary disease (COPD).
4) Fall
A fall was defined as a state where the body contacted the ground as a result of an unintentional falling. Fall frequency was defined as the number of fall downs experienced over the previous six months.
5) Self-assessed depressive symptoms
The presence of self-assessed depressive symptoms was determined based on responses to the question, "Have you felt depressed or sad during the past month?"
6) Covariates
The covariates included in the analysis were sociodemographic and psychophysical confounders affecting cognitive function,23,24,25,26) that is, gender, age, height, weight, BMI, blood pressure, hospitalization over the previous year, polypharmacy (the use of four or more regular medications), alcohol consumption (history of alcohol drinking in the past one year), smoking (Ever smoker [current or former] / never smoker), cohabiting family members, and years of education.
3. Statistical Analysis
Univariate analysis and multiple linear regression analysis were conducted to examine relations between demographics, CHS Frailty Indices, and K-MMSE scores (dependent variable). The chi-square test, the t-test, and one-way analysis of variance were used for univariate analysis. Correlates were then entered into a multiple regression model. Multiple linear regression analysis included an examination of interaction effects and assessments of possible multicollinearities among independent variables. PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
RESULTS
1. General Characteristics
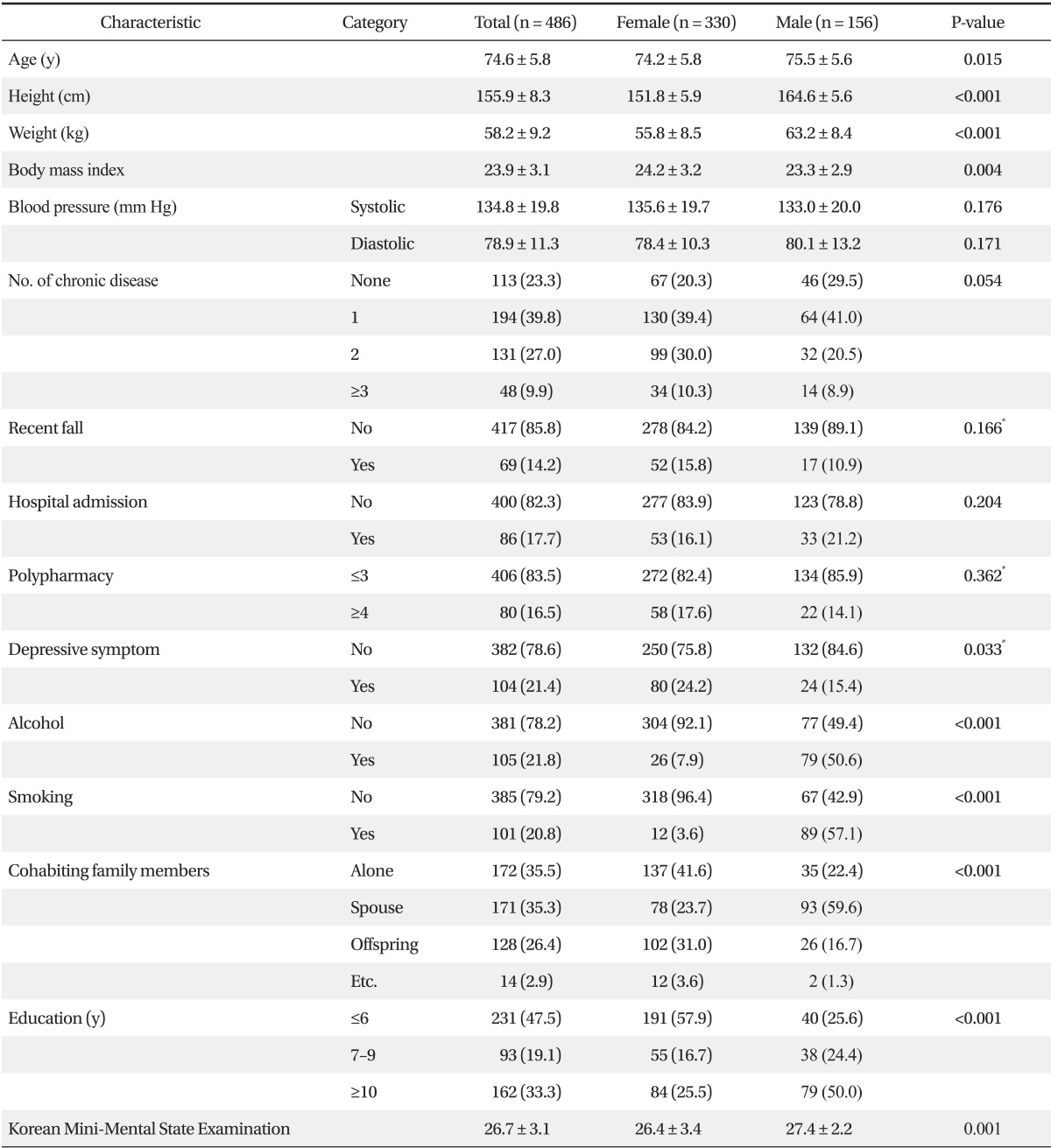
Mean ages were 75.5 ± 5.6 years for males and 74.2 ± 5.8 years for females. Males were taller and heavier than females (164.6 ± 5.6 cm and 63.2 ± 8.4 kg for males versus 151.8 ± 5.9 cm and 55.8 ± 8.5 kg for females, P < 0.001), but females had a greater mean BMI (24.2 ± 3.2 kg/m2 for males versus 23.3 ± 2.9 kg/m2 for females, P = 0.004). Smoking, drinking, and educational level were higher for males than females, but depression was more frequent in females (15.4% for males and 24.2% for females, P = 0.033). In terms of cohabiting family members, more males lived with a spouse (59.6% for males and 23.7% for females, P < 0.001), whereas more females lived with their offspring or alone. Mean K-MMSE scores were 27.4 ± 2.2 for males and 26.4 ± 3.4 for females, which was a significant difference (P = 0.001). No significant gender differences were found for blood pressure, number of chronic diseases, fall frequency over the previous six months, hospitalization over the previous year, or polypharmacy (Table 1).
2. The Cardiovascular Health Study Frailty Indices
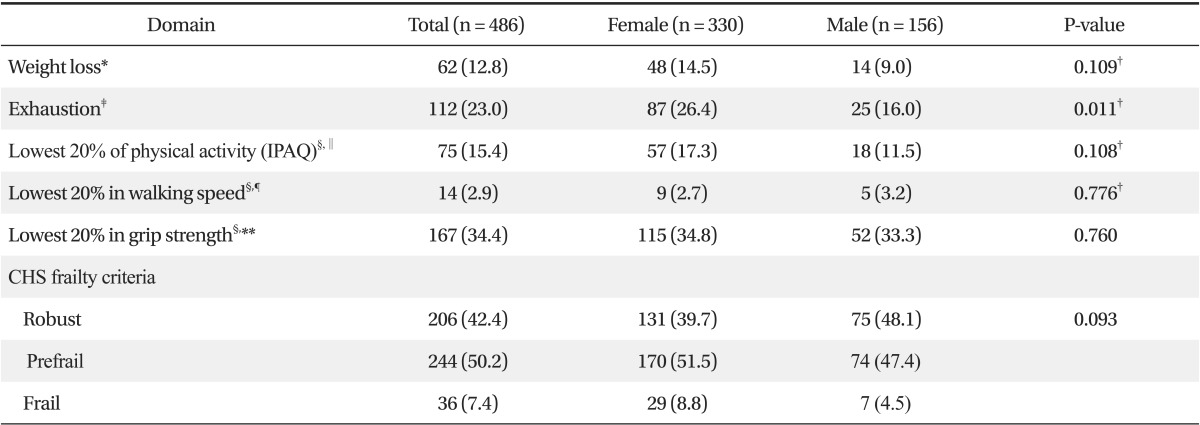
According to the CHS Frailty Indices, 36 subjects (7.4%) were frail, 244 subjects (50.2%) were prefrail, and 206 subjects (42.4%) were robust. No significant gender-associated differences were found (Table 2).
3. Factors that Affected Cognitive Function
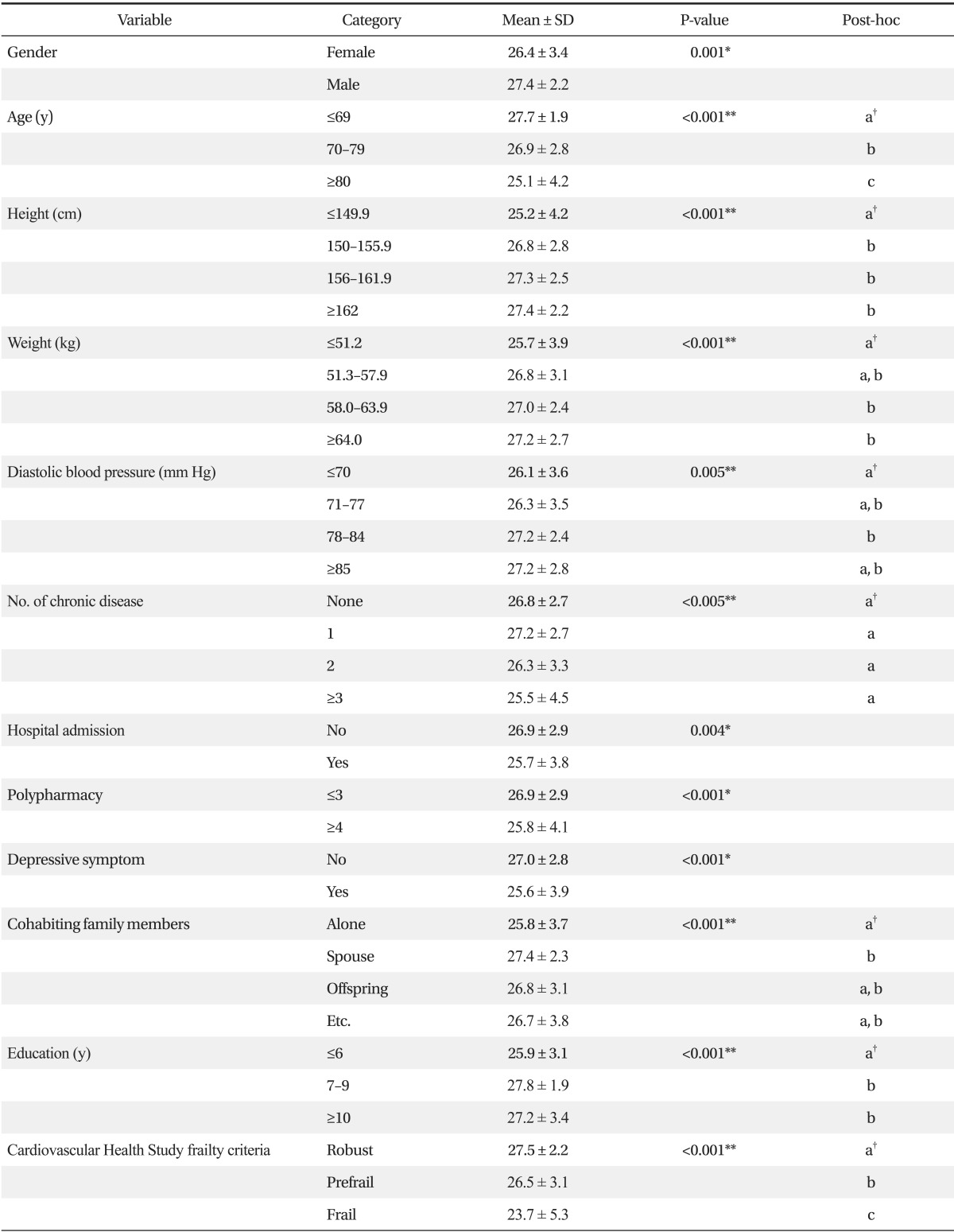
The factors found to affect K-MMSE scores were; gender, age, height, weight, diastolic blood pressure, number of chronic diseases, history of hospital admission, polypharmacy, self-assessed depression, cohabiting family members, education, and CHS Frailty Index. Mean K-MMSE score was higher for males (27.4 ± 2.2 for males and 26.4 ± 3.4 for females, P = 0.001) and scores significantly decreased with age (27.7 ± 1.9 for ≤69 years, 26.9 ± 2.8 for 70 to 79 years, and 25.1 ± 4.2 for ≥80 years; P < 0.001). Furthermore, subjects with a height of <150 cm and subjects with a weight of <51.3 kg had lower mean K-MMSE scores (P < 0.001 and P = 0.001, respectively). Although mean K-MMSE scores were not significantly affected by each chronic disease, such as MI, angina, CHF, PVD, arthritis, cancer, diabetes, hypertension, or COPD, number of chronic disease was related with K-MMSE (P = 0.005). Mean K-MMSE score was higher for subjects with a diastolic blood pressure (BP) of ≥78 mm Hg than for those with a diastolic BP of <71 mm Hg (P = 0.005). Mean K-MMSE score was found to be significantly lower for study subjects with a medical history of hospitalization within the previous six months (P = 0.004) and for those taking at least four medications (P = 0.001). Mean K-MMSE score was significantly lower in study subjects with self-assessed depression (P < 0.001), and in those living alone than in those living with a spouse (P = 0.001). Furthermore, mean K-MMSE score was found to be significantly lower for those that spent less than six years in full-time education (P < 0.001). In addition, difference was shown depend on the groups according to CHS frailty score. Mean K-MMSE score was 27.5 ± 2.2 in the robust group, 26.5 ± 3.1 in the pre-frail, and 23.7 ± 5.3 in the frail group (P < 0.001) (Table 3). Furthermore, when cognitive impairment was defined as a K-MMSE score of below 23 (≤23) points, 6.3% of robust subjects (13 of 206), 16.8% of prefrail subjects (41 of 244), and 30.6% of frail subjects (11 of 36) were found to be cognitive impaired (P < 0.001) (Figure 2).
4. Relationship between Cardiovascular Health Study Frailty Indices and Korean Mini-Mental State Examination Scores
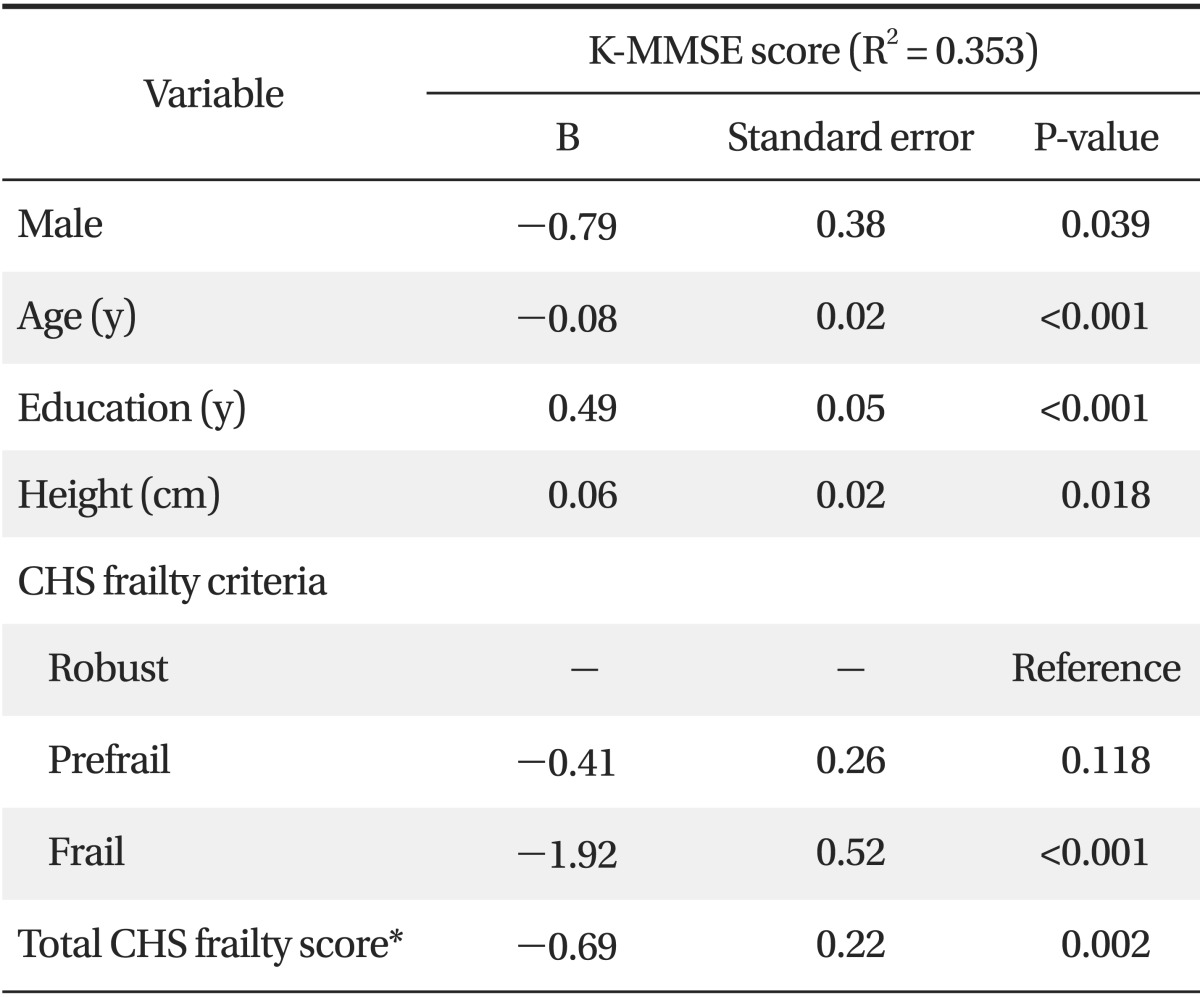
Multiple regression analysis was conducted using factors found to be significantly related to cognitive function by univariate analysis to prevent confounding effects. K-MMSE score was defined as the dependent variable, and gender, age, height, weight, diastolic blood pressure, number of chronic diseases, history of hospital admission, polypharmacy, self-assessed depression, family member, education, and CHS frailty criteria were analyzed as independent variables. Frailty was found to be negatively correlated with K-MMSE scores after adjustment for possible confounders (B = -1.92, SE 0.52; P < 0.001). However, no association was found between prefrailty and K-MMSE scores. When we also performed multiple regression analysis using total CHS frailty scores as a continuous independent variable instead of CHS frailty criteria, total CHS frailty scores were negatively correlated with K-MMSE scores (B = -0.69, SE 0.22; P = 0.002) (Table 4).
DISCUSSION
In this study, physical frailty and cognitive function were found to be negatively corelated in older Koreans (>65 years old) by multiple linear regression analysis after adjusting for other factors, which is in accord with the findings of previous studies.7,27) However, prefrailty was not found to be associated with lower K-MMSE scores (Table 4). Nevertheless, the prevalence of cognitive impairment (K-MMSE ≤ 23) among prefrail older adults (16.8%) was higher in comparison to robust older adults (6.3%) and substantially lower in comparison to frail older adults (30.6%) (Figure 2). Furthermore, when we performed multiple linear regression analysis to examine association total CHS frailty scores (as a continuous independent variable) with K-MMSE scores, they were also found to be negatively correlated (B = -0.69, SE 0.22; P = 0.002) (Table 4). The above mentioned results implicate that the prevention of transition to poorer frail status is important. Espinoza et al. emphasized the significance of prefrailty as a separate risk state because in their longitudinal study, substantially lower mortality was observed in prefrail than in frail individuals.28) The prefrail individuals are more likely to improve over time than frail individuals, and thus, may be responsive to clinical interventions to slow or reverse worsening of frailty.28) A recent study conducted in Hong Kong on transition between frailty states among Asians demonstrated that among prefrail older adults at baseline (850 males and 884 females), 23.4% of males and 26.6% of females improved after 2 years, and 11.1% of males and 6.6% of females worsened.29) In the study, a higher MMSE score was found to be protective among prefrail men, which means cognitive impairment indicates future frailty.29) This result supports the 'cycle of frailty' hypothesis, which states that frailty may occur as a result of chronic disease, immobility, malnutrition, depression, or dementia, and that frailty may in turn aggravate causal diseases.4,30,31,32) However, further longitudinal studies are required to identify the cause-effect relationships between frailty and cognitive function.
Although evidence regarding the effects of interventions targeting frailty coupled with cognitive decline is limited, a small number of studies have shown physical activity confers cognitive benefits.3) Landi et al.33) found that physical activity protected against both sarcopenia and cognitive decline in experimental training trials and in observational studies. Furthermore, a recent study demonstrated that a 12-week aerobic exercise and strength training program for frail and non-frail older adults improved functional capacity, physical endurance, cognition, and quality of life.34) In addition, balance exercises like Tai Chi have been reported to prevent frailty and inhibit its progression in older patients that found muscular strength exercises difficult.35) Further studies of interventions are needed to confirm that physical activity is beneficial to older people with frailty and cognitive impairment.
The prevalence of frailty in the present study (7.4%) was similar to that reported in previous studies.4,10,27) Some studies have reported that there were different prevalence of frailty among different ethnicities.10) However, in one study, when the cut-off values of the domains of the CHS Frailty Indices were modified for ethnic groups, prevalence of frailty were similar.36) Nevertheless, it should be noted that studies differ with respect to the prevalence of frailty in each domain of the CHS Frailty Indices.4,10,27) In the present study, a much lower prevalence of frailty was observed in 'reduced gait speed' domain (2.9%) than in other studies (19.9% in Mexican Americans10) and 20.0% in European Americans4)). On the other hand, the 34.4% prevalence of reduced grip strength was higher than in other studies (15.4% in Mexican Americans10) and 20.0% in European Americans4)). These differences might have been caused because our study population (n = 486) did not represent the normal older population. For this reason, we adopted cutoff values of the lowest 20% as determined by a large sized study (survey on health and welfare status of the elderly in Korea 2008).22) On the other hand, in previous studies cutoff values of the lowest 20% in their study populations were used (n = 5,317 in Fried et al.'s study4) and n = 1,370 in Samper-Ternent et al.'s study10)), which could represent their normal older populations. Hence, a small number of subjects exhibited reduced gait speed. In addition, we did not consider measurement bias coming from different walk distance to calculate gait speed between our study (4.5 m) and the reference study (2.5 m).22)
On the other hand, the prevalence of reduced grip strength (34.3%) was higher in the present study than in other studies (15.4% in Mexican Americans10) and 20.0% in European Americans4)). It means that grip strength was lower in our study population than in the normal older population. Because grip strength declines with age,30) this phenomenon could be explained by age distribution. In our study population, there was a lower proportion of subjects aged ≤ 70 years (27.7% vs. 38.0%) and a higher proportion of aged ≥ 80 years old (25.1% vs. 16.0%) than in the normal older population.22) Furthermore, there is a possibility for bias from different tools used to measure grip strength. The JAMAR hydraulic hand dynamometer (Sammons Preston) was used in our study, but the TANITA Hand Grip Meter Bule 6103 (Tanita Co.) was used in the reference study (Survey on health and welfare status of the elderly in Korea 2008).22)
The prevalence of frailty depends on the domain cutoff values used for physical activity, gait speed, grip strength, and others. The most appropriate cutoff values for reduced gait speed and reduced grip strength are important issues that remain to be resolved in the sarcopenia field. The guidelines issued by the European Working Group on Sarcopenia in Older People in 2010 suggest a cutoff value of <0.8 m/s be used for low gait speed, and that cutoff values of <30 kg for males and <20 kg for females be used to determine reduced grip strength.37) A large scale of study on sarcopenia in the US, Foundation for the National Institutes of Health (FNIH) Sarcopenia Project conducted in 2014 recommended cutoff values for grip strength < 26 kg for male and <16 kg for female.38) However, the study population of the FNIH Sarcopenia Project was predominantly Caucasian (90%), and thus, it is questionable whether it can be applied to other different ethnicities. Further studies are required to determine cutoff values with high sensitivity and specificity to screen for frailty and sarcopenia.
Limitations of this study required consideration. First, the representativeness of study populations recruited from community senior welfare centers is questionable. However, we used representative cutoff values obtained by reanalyzing data of a large number study (survey on health and welfare status of the elderly in Korea 2008).22) Second, we were not able to investigate cause-effect relationships between frailty and cognitive function due to the cross-sectional nature of the study. Third, self-assessed depression might be inaccurate as no physician assessments were performed, and depression is an important consideration because it can mimic cognitive impairment. Fourth, we analyzed only total K-MMSE scores, but some study assessed that association frailty with the each domain of MMSE and reported that being frail was related with worse performance in domains of MMSE such as time orientation, immediate memory, and the ability to follow commands.27) Nevertheless, the present study is the first study to describe the association between frailty and cognitive function in older Koreans.
In conclusion, this study shows that frailty is associated with cognition impairment in older Koreans. However, prospective cohort studies are required to provide higher levels of evidence.
ACKNOWLEDGEMENTS
We would like to thank The Senior Functional Assessment Research Group of The Korean Geriatrics Society for collecting the data.
Notes
No potential conflict of interest relevant to this article was reported.