 |
 |
- Search
| Korean J Fam Med > Volume 36(6); 2015 > Article |
Abstract
Background
Methods
Results
References
Table┬Ā1
General characteristics of study subjects

Values are presented as mean┬▒standard error or number (%). P-values were calculated by Student t-test or chi-square test. uACR, glucose, TG, HDLC, AST, ALT, and GGT were log transformed because they were not normally distributed. The data describing medications and life style were obtained from structured questionnaires. The eGFR (mL/min/1.73 m2)=186├Śserum creatinine (mg/dL)-1.154├Śage-0.203├Ś0.742 (if female).
HTN, hypertension; DM, type 2 diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; uACR, urine albumin-to-creatinine ratio; HbA1c, hemoglobin A1c; TG, triglyceride; HDLC, high density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase.
*HTN was defined as having a SBP of 140 mm Hg or higher, or a DBP of 90 mm Hg or higher. DM was defined as having a fasting glucose of 126 mg/dL or higher, or a HbA1c of 6.5% or higher.
Table┬Ā2
Correlation analysis for uACR according to gender and diseases (HTN or DM)
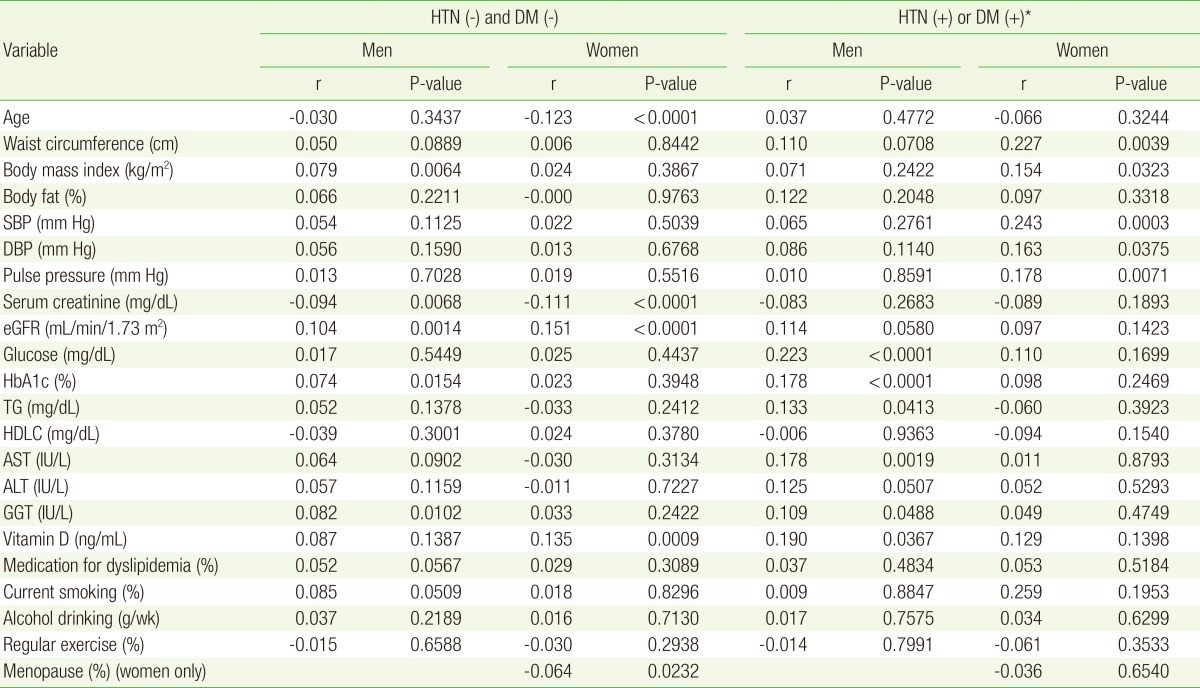
uACR, glucose, TG, HDLC, AST, ALT, and GGT were log transformed because they were not normally distributed. The data describing medications and life style were obtained from structured questionnaires. The eGFR (mL/min/1.73 m2)=186├Śserum creatinine (mg/dL)-1.154├Śage-0.203├Ś0.742 (if female).
uACR, urine albumin-to-creatinine ratio; HTN, hypertension; DM, type 2 diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; TG, triglyceride; HDLC, high density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase.
*HTN was defined as having a SBP of 140 mm Hg or higher, or a DBP of 90 mm Hg or higher. DM was defined as having a fasting glucose of 126 mg/dL or higher, or a HbA1c of 6.5% or higher.
Table┬Ā3
Multiple linear regression analysis

Urine albumin-to-creatinine ratio, glucose, TG, and GGT were log transformed because they were not normally distributed. The data describing life style were obtained from structured questionnaires. Values are standardized ╬▓-coefficients. The eGFR (mL/min/1.73 m2)=186├Śserum creatinine (mg/dL)-1.154├Śage-0.203├Ś0.742 (if female).
HTN, hypertension; DM, type 2 diabetes mellitus; SBP, systolic blood pressure; HbA1c, hemoglobin A1c; TG, triglyceride; GGT, gamma-glutamyl transpeptidase; eGFR, estimated glomerular filtration rate.
*HTN was defined as having a SBP of 140 mm Hg or higher, or a DBP of 90 mm Hg or higher. DM was defined as having a fasting glucose of 126 mg/dL or higher, or a HbA1c of 6.5% or higher.
- TOOLS
-
METRICS

- Related articles in KJFM
-
Fruit Intake to Prevent and Control Hypertension and Diabetes2021 January;42(1)





