|
 |
- Search
| Korean J Fam Med > Volume 43(6); 2022 > Article |
|
Abstract
Background
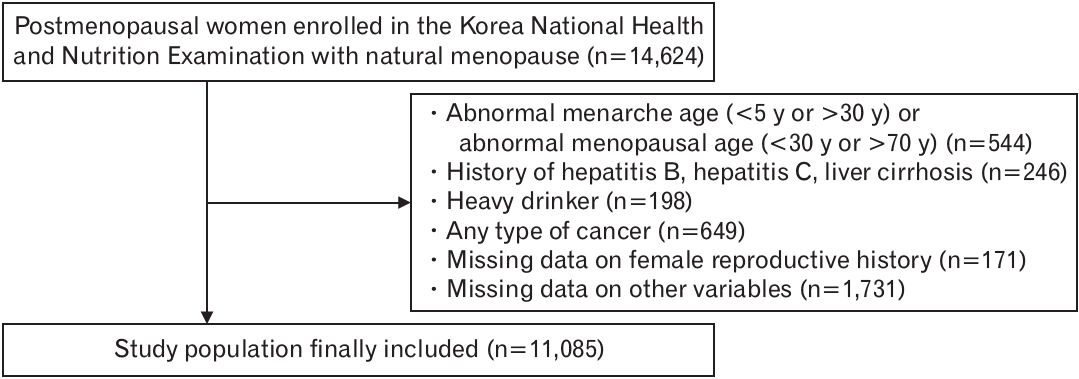
Methods
Results
Notes
FUNDING
This research was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Korean government, Ministry of Science, and ICT (2020R1A2C1099826), as well as a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Republic of Korea (2022R1I1A1A01054327).
Table 1.
| Characteristic |
Fibrosis-4 index score |
P-value | ||
|---|---|---|---|---|
| <2.67 | ≥2.67 | |||
| No. of participants | 10,713 | 372 | ||
| Demographics | ||||
| Age (y) | 62.6±0.1 | 70.9±0.5 | <0.0001 | |
| Age (y) | <0.0001 | |||
| <50 | 3.62 (0.3) | 1.38 (0.9) | ||
| 50–59 | 40.3 (0.6) | 13.7 (2.2) | ||
| 60–69 | 31.2 (0.5) | 18.4 (2.2) | ||
| 70–79 | 20.5 (0.5) | 51.0 (2.9) | ||
| ≥80 | 4.4 (0.2) | 15.7 (2.1) | ||
| Alcohol consumption* | 26.9 (0.5) | 18 (2.4) | 0.001 | |
| Current smoking | 3.69 (0.3) | 2.8 (1.0) | 0.410 | |
| Education (≥high school graduates) | 30.9 (0.7) | 18.0 (2.5) | <0.0001 | |
| Low income | 30.1 (0.6) | 47.2 (3.0) | <0.0001 | |
| Height (cm) | 153.9±0.1 | 151.7±0.4 | <0.0001 | |
| Weight (kg) | 57.4±0.1 | 55.2±0.51 | <0.0001 | |
| Waist circumference (cm) | 81.9±0.1 | 82.3±0.6 | 0.546 | |
| BMI (kg/m2) | 24.2±0.04 | 24.0±0.2 | 0.206 | |
| BMI (kg/m2) | ||||
| <18.5 | 1.9 (0.2) | 4.5 (1.2) | ||
| 18.5–22.9 | 35.9 (0.6) | 35.6 (2.9) | ||
| 23.0–24.9 | 25.6 (0.5) | 23 (2.6) | ||
| ≥25.0 | 36.5 (0.6) | 36.9 (2.9) | ||
| Urban residence† | 23.9 (1.1) | 26.1 (3.0) | 0.429 | |
| Past medical history | ||||
| Diabetes mellitus | 12.2 (0.4) | 16.8 (2.4) | 0.030 | |
| Hypertension | 35.7 (0.6) | 46.0 (3.1) | 0.001 | |
| Dyslipidemia | 18.7 (0.5) | 18.5 (2.4) | 0.951 | |
| Cardiovascular disease | 3.2 (0.2) | 7.0 (1.6) | 0.001 | |
| Chronic kidney disease | 0.3 (0.1) | 0.6 (0.5) | 0.365 | |
| Stroke | 1.8 (0.1) | 4.0 (1.0) | 0.003 | |
| Thyroid disease | 3.8 (0.2) | 2.6 (0.9) | 0.256 | |
| Laboratory findings | ||||
| Fasting blood glucose (mg/dL) | 101.8±0.3 | 103.0±1.6 | 0.441 | |
| Systolic blood pressure (mm Hg) | 124.5±0.2 | 128.7±1.0 | <0.0001 | |
| Diastolic blood pressure (mm Hg) | 75.7±0.1 | 73.4±0.7 | 0.002 | |
| Total cholesterol (mg/dL) | 201.6±0.4 | 183.3±2.3 | <0.0001 | |
| Triglyceride (mg/dL) | 116 (115–118) | 104 (98–110) | 0.0003 | |
| High-density lipoprotein cholesterol (mg/dL) | 51±0.1 | 49.2±0.9 | 0.077 | |
| Aspartate transaminase (IU/L) | 21.5 (21.3–21.6) | 34.0 (31.7–36.4) | <0.0001 | |
| Alanine aminotransferase (IU/L) | 17.9 (17.7–18.1) | 21.6 (19.8–23.7) | <0.0001 | |
| Creatinine (mg/dL) | 0.74±0 | 0.8±0.01 | 0.015 | |
| Reproductive factors | ||||
| Age at menarche (y) | 15.5±0.03 | 15.9±0.1 | 0.003 | |
| Age at menarche (y) | 0.033 | |||
| <12 | 1.2 (0.1) | 0.4 (0.3) | ||
| 12–13 | 15.2 (0.5) | 12.4 (2.0) | ||
| 14–15 | 33.7 (0.6) | 29.3 (2.7) | ||
| ≥16 | 49.9 (0.6) | 57.8 (3.0) | ||
| Age at menopause (y) | 49.7±0.1 | 49.2±0.3 | 0.045 | |
| Age at menopause (y) | 0.0003 | |||
| <45 | 10.1 (0.3) | 13.9 (1.9) | ||
| 45–49 | 32.7 (0.5) | 39.0 (2.9) | ||
| 50–54 | 47.1 (0.6) | 34.4 (2.9) | ||
| ≥55 | 10.1 (0.3) | 12.7 (2.1) | ||
| Total reproductive years (y) | 34.2±0.1 | 33.3±0.3 | 0.004 | |
| Total reproductive years (y) | 0.004 | |||
| <30 | 13.3 (0.4) | 20.0 (2.3) | ||
| 30–34 | 34.8 (0.6) | 36.8 (2.9) | ||
| 35–39 | 41.5 (0.6) | 33.5 (2.8) | ||
| ≥40 | 10.4 (0.4) | 9.7 (1.8) | ||
| Oral contraceptives use | 0.487 | |||
| None | 78.6 (0.5) | 80.4 (2.4) | ||
| Yes | 21.4 (0.5) | 19.6 (2.4) | ||
| No. of pregnancies | 0.0003 | |||
| None | 1.2 (0.1) | 3.1 (1.0) | ||
| 1 | 2.4 (0.2) | 1.1 (0.6) | ||
| 2 | 11.0 (0.4) | 6.1 (1.3) | ||
| ≥3 | 85.4 (0.4) | 89.6 (1.7) | ||
Table 2.
Ref, reference; Q1, the lowest quartile; Q4, the highest quartile.
Liver fibrosis was defined as Fibrosis-4 index score more than 2.67 kg/m2 in women. Model 1: adjusted for age; model 2: additionally adjusted for income, education, alcohol consumption, smoking, and urban residence; model 3: additionally adjusted for comorbidities (diabetes, hypertension, dyslipidemia, cardiovascular disease, stroke, chronic kidney disease, and thyroid disease); and model 4: additionally adjusted for body mass index.
Table 3.
REFERENCES
- TOOLS