 |
 |
- Search
| Korean J Fam Med > Volume 44(1); 2023 > Article |
|
See commentary "Does Changing the Ratio of Dietary Sodium-to-Potassium Intake Affect Bone Mineral Density?" in Volume 44 on page 1.
Abstract
Background
Dietary Na+ or Na+/K+ ratio has been reported to be associated with bone mineral density (BMD). However, this remains unclear, and only a few studies have been reported on the Korean population. Therefore, this study aimed to determine the association between dietary Na+, K+, and Na+/K+ ratios and BMD in middle-aged Korean women.
Methods
This study used data from the Korea National Health and Nutrition Examination Survey 2008–2011. A total of 3,690 women aged >50 years were included. Study participants were classified into quartiles (lowest quartile Q1–highest quartile Q4) according to dietary Na+, K+, and Na+/K+ ratio, and we examined the association of these parameters with BMD. Total femur and lumbar spine BMD were measured using dual-energy X-ray absorptiometry. Multiple linear regression analyses were performed using IBM SPSS ver. 19.0.
Results
The mean age was 62 years, and a significant negative trend in the β-coefficient regarding dietary Na+ was only observed in the total femur BMD. However, the total femur and lumbar spine BMD decreased from Q1 to Q4 regarding the dietary Na+/K+ ratio (P-value for trend: 0.044 for total femur BMD and 0.002 for lumbar spine BMD).
According to the World Health Organization, osteoporosis is a systemic skeletal disease characterized by decreased bone mass and microstructural abnormalities, resulting in a fragile state due to bone weakness [1]. Bone fractures are the most serious complication of osteoporosis, and the condition itself is serious, causing death in some cases. Among hip fracture patients aged ≥50 years, 24% die within the year due to a fracture [2]. Of nearly 300,000 people with a hip fracture each year, 25% end up in nursing homes, and 50% never regain their previous function [3]. Women’s risk of fractures is equal to their combined risk of breast, uterine, and ovarian cancer [4]. Osteoporosis results in a substantial economic burden to healthcare systems, totaling more than US $17 billion. Experts predict osteoporosis will be responsible for three million fractures, resulting in $25.3 billion in costs by 2025 [5]. Osteoporosis can be prevented, diagnosed, and treated before the occurrence of fractures. All postmenopausal women can benefit from nonpharmacological interventions to reduce the risk of fracture, including a balanced diet with adequate intake of calcium and vitamin D, regular exercise, measures to prevent falls or minimize their impact, smoking cessation, and moderation of alcohol intake [6].
Among the many factors affecting bone density, the role of some nutrients, such as calcium and vitamin D [7], in bone health is obvious, but the effects of other nutrients are less well known. Some studies have reported that certain dietary factors, such as sodium, may modulate calcium metabolism. High sodium consumption can affect bone density due to a negative calcium balance, and induced hypercalciuria can play an important role in inducing osteoporosis [8]. A study that used data from 2008 to 2011 National Health and Nutrition Survey found a link between sodium intake and osteoporosis in postmenopausal women. The study showed that the prevalence of osteoporosis in the lumbar spine was significantly higher in participants who consumed ≥4,001 mg of salt than that in those who consumed ≤2,000 mg of salt. At the femoral neck, the rates were significantly higher for those who consumed ≥5,001 mg than that for those who consumed ≤4,000 mg [9]. However, studies on the effect of dietary/urinary sodium on osteoporosis are controversial [10,11]. A systematic review concluded that the association of dietary/urinary sodium with bone mineral density (BMD) demonstrated a positive association between sodium intake and the risk of osteoporosis, whereas no association was found with urinary sodium [12]. Conversely, high potassium intake is considered beneficial for the skeleton because it improves calcium and phosphorus balance, reduces bone resorption, and increases the rate of bone formation [13]. Low potassium and high sodium dietary intakes have been reported to be associated with accelerated bone resorption in both animal and population-based studies [14,15]. A recent cross-sectional study reported that urinary sodium-to-potassium (Na+/K+), but not individual values of sodium or potassium, was inversely related to BMD in the whole body, total hip, trochanter, and intertrochanter in women [16]. To the best of our knowledge, information currently available regarding the role of the dietary Na+/K+ ratio in BMD is limited. To address this issue, this community-based study aimed to determine the associations between dietary sodium, dietary potassium, and Na+/K+ ratio with BMD in women aged >50 years.
The Centers for Disease Control and Prevention has conducted the Korea National Health and Nutrition Examination Survey (KNHANES) every 3 years since 1998 to produce representative and reliable statistics on the overall health, nutritional status, and trends of the people [17]. With the 4th and 5th KNHANES (2007–2012), the participants were recruited between 2008 and 2011, as there were BMD results only in 2008–2011, with an average response rate of 75%–80%. Among the 37,753 participants who participated in the KNHANES 2008–2011, we included women aged 50 years and older who answered the dietary questionnaires and completed body composition measurements. Among these participants, we excluded those with any diseases that could affect bone density, such as asthma, liver disease, chronic kidney disease, and cancers. Finally, 3,690 participants were included in this study (Figure 1). The institutional review board of Daegu Catholic University Medical Center reviewed and approved this study (approval no., CR-21-108).
BMD (g/cm2) was measured using dual-energy X-ray absorptiometry (Hologic Discovery; Hologic Inc., Bedford, MA, USA) using the standard techniques of the Korean Society of Osteoporosis and Hologic Discovery software ver. 13.1 (Hologic Inc.). Total femur and lumbar spine BMDs were used for analysis.
A 24-hour dietary recall questionnaire and 63 food-items were investigated for dietary assessment. The frequency of food consumption was based on the dietary habits of the past 1 year. We used the daily energy intake and intake of other nutrients, including sodium and potassium, which were calculated using a food frequency questionnaire. The study participants were divided into four groups according to their daily sodium intake, potassium intake, and Na+/K+ ratio.
Height and weight were measured to the nearest 0.1 cm for height and 0.1 kg for weight, respectively, using standard protocols. Body mass index (BMI) was calculated as body weight divided by the square of height in meters, as presented in the original data. Lifestyle factors were assessed using self-administered questionnaires.
Physical activity was assessed based on the frequency and intensity of exercise during the past 1 week. Low-intensity exercise was defined as walking more than 5 days per week for more than 30 minutes at a time. Moderate-to-vigorous-intensity exercise was defined as participating in moderate-intensity activities for more than 5 d/wk for more than 30 minutes at a time or participating in vigorous-intensity activities for more than 3 d/wk for more than 20 minutes at a time. Smoking status was categorized as never smoked or ever smoked. Never smokers were defined as those who had never smoked or had smoked fewer than 100 cigarettes in their lifetime. Ever-smokers were defined as those who had smoked more than 100 cigarettes in their lifetime. High-risk alcohol consumption was defined as drinking alcohol more than twice per week, with an average amount per drink ≥7 glasses for men and ≥5 glasses for women. Menopausal status was assessed based on the self-reported questionnaire.
We used sampling weights based on the complex sample design of the KNHANES in all analyses. Continuous variables were presented as means and standard errors, and discrete variables were presented as numbers with proportions. Since BMD, as an outcome variable, is a continuous variable, we used simple and multiple linear regression analyses. Simple linear regression analyses were performed to determine the clinical variables adjusted for multiple linear regression analyses. Study participants were classified into quartiles (lowest quartile Q1–highest quartile Q4) according to dietary Na, K, and Na/K ratios, and the association of these parameters with BMD was examined thereafter. Multiple linear regression analyses were performed to examine these associations, and the results are shown as β-coefficients. We adjusted for age, BMI, physical activity, smoking, alcohol consumption, menopausal status, serum 25-hydroxyvitamin D (25(OH)D) level, and daily intake of total energy, protein, calcium, and phosphorus [18,19]. BMD values were log-transformed to approximate normal distribution for the analyses. IBM SPSS software for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for all the analyses. Statistical significance was set at P<0.05.
The baseline characteristics of the 3,690 participants according to quartiles of dietary Na+/K+ ratios are shown in Table 1. There were no significant differences in age or weight, although BMI tended to increase as the dietary Na+/K+ ratio increased. Regarding BMD, both the total femur and lumbar spine BMD tended to decrease as the dietary Na+/K+ ratio increased. There were no significant differences in total energy, protein, and fat intake among the groups according to the dietary Na+/K+ ratio. However, carbohydrate, fiber, and phosphorus intake decreased as the dietary Na+/K+ ratio increased. There was no difference in calcium intake between the groups. Regarding lifestyle factors, such as smoking, alcohol consumption, and physical activity, there were no differences according to the dietary Na+/K+ ratio.
Table 2 shows simple linear regression analyses of BMD and clinical variables. Height, weight, BMI, calcium, phosphorus, total energy, and protein intake were positively associated with both total femur and lumbar spine BMD. Age was negatively associated with BMD. Serum 25(OH)D was not significantly associated with both femur and lumbar spine BMD.
Tables 3 and 4 show the adjusted association of dietary Na+ intake, K+ intake, and Na+/K+ ratio with the total femur and lumbar spine BMD. As shown in Table 3, the β-coefficient for total femur BMD in Q4 of Na+ intake was -0.012, although the difference was not statistically significant. In Q3 and Q4 of K+ intake, the β-coefficients for total femur BMD were 0.004 and 0.009, respectively, although statistical significance was not observed. However, the lumbar spine BMD significantly decreased with dietary Na+. Regarding dietary Na+, the β-coefficient (95% confidence interval) was -0.007 (-0.031 to 0.017) in Q2, -0.012 (-0.036 to 0.013) in Q3, and -0.031 (-0.058 to -0.004) in Q4 (P=0.023). This means that each group had a lower BMD by 0.7%, 1.2%, and 3.1%, respectively, compared with that of Q1 by applying the exponential function to the presented β, although statistical significance was only observed in Q4.
As shown in Table 4, there were significant negative associations among the Na+/K+ ratio, total femur BMD, and lumbar spine BMD. The β-coefficient (95% confidence interval) for total femur BMD was -0.021 (-0.037 to -0.005) in Q2, -0.009 (-0.025 to 0.007) in Q3, and -0.023 (-0.042 to -0.005) in Q4 (P=0.044). This means that each group had a lower BMD of 2.1%, 0.9%, and 2.3%, respectively, compared to that of Q1, although statistical significance was not observed in Q3. The β-coefficient (95% confidence interval) for the lumbar spine was -0.020 (-0.041 to 0.002) in Q2, -0.027 (-0.046 to -0.008) in Q3, and -0.033 (-0.055 to -0.012) in Q4 (P=0.002). This means that each group had a lower BMD of 2.0%, 2.7%, and 3.4%, respectively, compared with that of Q1, although statistical significance was not observed for Q2.
This community-based study in Korean women showed an independent and inverse association between dietary Na+/K+ ratio and BMD of the lumbar spine and total femur after adjustment for multiple covariates. Total femur BMD was significantly decreased according to dietary Na+/K+ quartiles, and lumbar spine BMD was significantly decreased according to dietary sodium and Na+/K+ quartiles. No significant association was found between dietary potassium intake and the BMD of the lumbar spine or total femur. These results showed that the total femur and lumbar spine BMD tended to decrease as the dietary Na+/K+ ratio increased.
Many studies have reported associations between the Na+/K+ ratio and risk factors related to cardiovascular disease (CVD) in the general population [20-23]. A higher urinary Na+/K+ ratio is also associated with an increased risk of subsequent CVD [24]. However, only a few studies have focused on the combined effects of sodium and potassium on bone health. One community-based cross-sectional study showed that the urinary Na+/K+ ratio was inversely associated with BMD in the whole body, total hip, trochanter, and intertrochanter, but not the individual creatinine-adjusted values of sodium or potassium in women [16]. Consistent with previous studies, our study found that the dietary Na+/K+ ratio also had stronger inverse associations with BMD, supporting the hypothesis that the combined effect of these two electrolytes plays a role in the pathogenesis of chronic disease [25].
Several studies have reported that bone rebuilding varies inversely with the sodium intake [26,27]. Sodium intake elevates urinary calcium excretion and may lead to increased bone remodeling and loss [28]. The KNHANES 2008–2011, which used dietary Na+ intake to find a relationship with osteoporosis, found that participants with a higher sodium intake (≥4,001 mg) showed a significantly higher odds ratio (OR) for developing lumbar and femoral neck osteoporosis than those with a lower intake (≤2,000 mg) in Korean menopausal women (OR, 1.59; P=0.011) [9]. A meta-analysis summarizing the relationship between dietary sodium intake and BMD and the risk of osteoporosis found a positive association between dietary sodium intake and the risk of osteoporosis (OR, 1.20; P=0.026), whereas no association was found with urinary sodium intake. Furthermore, there is no significant correlation between sodium intake and BMD [12]. Dietary sodium exhibited significant inverse associations with BMD of the lumbar spine but not with BMD of the total femur in this study.
High dietary potassium intake is beneficial for the maintenance of bone mass [15]. In a prospective cohort study of elderly postmenopausal women, participants in the highest quartile of urinary potassium excretion had a significantly higher total hip BMD at 1 and 5 years [13]. In one interventional study, participants taking potassium citrate at 60 mEq/d showed a significant increase in lumbar BMD [29]. Conversely, a study of a 2-year trial in 276 postmenopausal women, comparing intake of high-dose potassium citrate, low-dose potassium citrate, placebo, and 300 g of additional fruit and vegetables/d, showed no beneficial effect on either bone turnover or BMD. In our study, we did not find any significant association between dietary potassium intake and BMD of the lumbar spine or the total femur. These results suggest that potassium does not improve bone health alone [30].
The possible mechanism for the favorable impact of dietary minerals on bone metabolism is based on the acid-base balance hypothesis [31]. Bone minerals function as a buffer base. In Western diets, high levels of meat and grain and low levels of fruits and vegetables may cause metabolic acidosis. As age increases, systematic metabolic acidosis worsens while renal function declines. Alkaline calcium salts in the skeleton cushion the acidic pH, resulting in bone loss. Therefore, alkaline potassium salts or supplements (potassium bicarbonate or potassium citrate excluding potassium chloride) produced by the metabolism of fruits and vegetables may protect against bone resorption via pH homeostasis [32].
The current study has some limitations. First, our results cannot establish causality between the Na+/K+ ratio and BMD, owing to the cross-sectional nature of the study. Therefore, longitudinal studies are required to establish the causality between the Na+/K+ ratio and BMD or osteoporosis. Second, dietary intake of sodium, potassium, and other nutrients was evaluated via a 24-hour dietary recall questionnaire, and the frequency of 63 food-items may not correspond to the participants’ usual intake. Lastly, we could not exclude the possibility of potential confounding variables, such as calcium or vitamin D supplementation, which have previously exhibited strong influences on BMD, even if we adjusted for the relevant confounders. Despite these limitations, this is the first study to identify the association between the dietary Na+/K+ ratio and BMD in middle-aged Korean women.
Our findings reveal an independent and inverse association between the dietary Na+/K+ ratio and BMD of the lumbar spine and total femur in Korean women aged 50 years or older. The association between BMD and combined sodium and potassium was stronger than that with either dietary sodium or potassium alone. These results highlight the importance of providing a good basis for recommending reduced salt intake, balanced Na+/K+ intake, and sufficient fruit intake to prevent osteoporosis in middle-aged women.
Figure. 1.
Flowchart of study participants. KNHANES, Korea National Health and Nutrition Examination Survey.
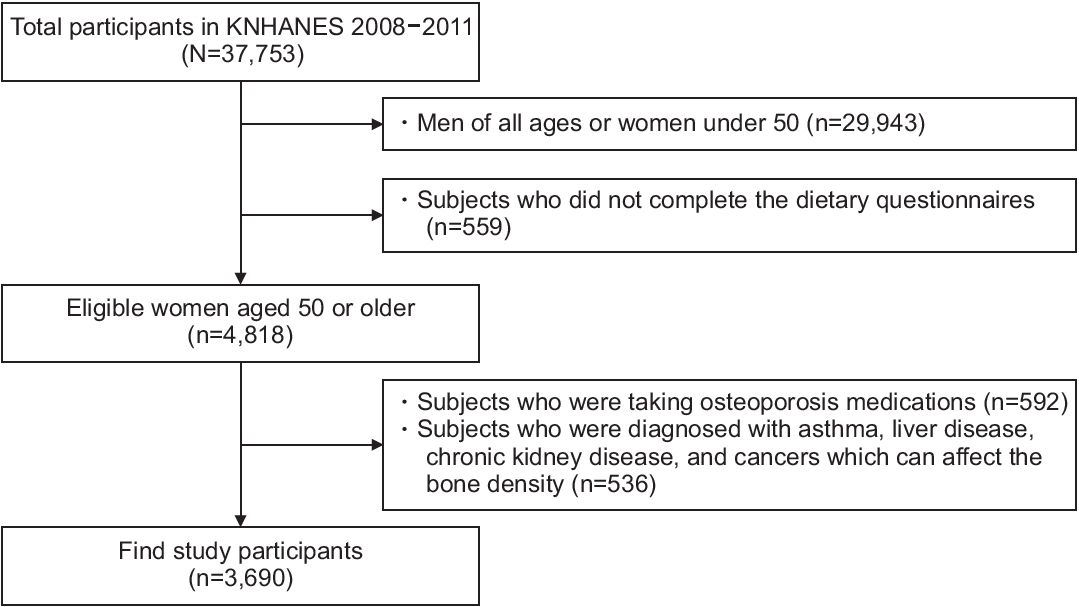
Table 1.
Baseline characteristics of study participants according to the quartiles of dietary sodium to potassium ratio* (N=3,690)
| Characteristic | Q1 | Q2 | Q3 | Q4 | P-value† | |
|---|---|---|---|---|---|---|
| Age (y) | 61.8±0.4 | 61.6±0.4 | 62.6±0.4 | 62.6±0.4 | 0.060 | |
| Height (cm) | 154.1±0.2 | 153.4±0.3 | 153.3±0.2 | 153.2±0.3 | 0.006 | |
| Weight (kg) | 57.2±0.3 | 56.6±0.4 | 57.2±0.4 | 57.2±0.4 | 0.673 | |
| Body mass index (kg/m2) | 24.1±0.1 | 24.0±0.1 | 24.3±0.1 | 24.4±0.1 | 0.037 | |
| Bone mineral density (mg/cm2) | ||||||
| Total femur | 798.4±4.5 | 786.7±5.5 | 783.1±4.7 | 779.7±5.5 | 0.011 | |
| Lumbar spine | 835.4±5.3 | 827.7±6.9 | 810.8±5.9 | 815.9±6.5 | 0.005 | |
| Energy intake (kcal/d) | 1,582.1±33.6 | 1,582.4±27.5 | 1,556.6±24.5 | 1,509.5±26.9 | 0.067 | |
| Protein intake (g/d) | 51.3±1.2 | 53.5±1.1 | 53.3±1.2 | 51.6±1.3 | 0.887 | |
| Fat intake (g/d) | 21.4±0.7 | 24.0±0.8 | 24.0±0.9 | 23.0±1.0 | 0.218 | |
| Carbohydrate (g/d) | 304.7±6.8 | 288.7±5.3 | 282.7±4.2 | 273.4±4.7 | <0.001 | |
| Fiber intake (g/d) | 8.6±0.3 | 7.0±0.2 | 6.3±0.2 | 6.3±0.2 | <0.001 | |
| Calcium intake (mg/d) | 413.4±11.2 | 437.2±12.2 | 417.3±13.2 | 427.0±24.5 | 0.802 | |
| Phosphorus intake (mg/d) | 997.4±20.8 | 970.7±17.9 | 940.1±17.3 | 891.1±19.0 | <0.001 | |
| Sodium intake (mg/d) | 2,214.2±52.6 | 3,273.8±72.9 | 3,949.2±88.3 | 5,778.9±165.9 | <0.001 | |
| Potassium intake (mg/d) | 3,214.9±74.5 | 2,671.6±60.5 | 2,382.5±52.8 | 2,206.9±56.6 | <0.001 | |
| Serum 25(OH)D (ng/mL) | 18.5±0.3 | 18.4±0.3 | 18.1±0.3 | 17.8±0.3 | 0.060 | |
| Ever smoker (%) | 5.9 (1.0) | 9.1 (1.5) | 8.6 (1.2) | 8.4 (1.3) | 0.242 | |
| High-risk alcohol consumption (%) | 0.2 (0.2) | 0.2 (0.1) | 0.6 (0.3) | 0.1 (0.1) | 0.194 | |
| Physical activity (%) | 0.590 | |||||
| None | 19.8 (2.0) | 19.1 (2.0) | 22.3 (2.3) | 24.0 (2.1) | ||
| Low intensity | 43.8 (2.4) | 46.5 (2.7) | 44.7 (2.8) | 41.7 (2.4) | ||
| Moderate to vigorous intensity | 36.4 (2.3) | 34.4 (2.7) | 33.1 (2.5) | 34.2 (2.5) | ||
| Menopause (%) | 83.0 (1.5) | 82.1 (1.7) | 79.6 (1.6) | 82.1 (1.6) | 0.457 | |
Table 2.
Simple linear regression of BMD† and clinical variables
| Variable | Total femur BMD† | Lumbar spine BMD† |
|---|---|---|
| Age (y) | -0.011*** | -0.010*** |
| Height (cm) | 0.011*** | 0.011*** |
| Weight (cm) | 0.009*** | 0.009*** |
| Body mass index (kg/m2) | 0.016*** | 0.016*** |
| Daily calcium intake (mg/d) | 5.753E-5* | 5.520E-5* |
| Daily phosphorus intake (mg/d) | 7.870E-5*** | 6.789E-5*** |
| Daily total energy intake (kcal/d) | 4.988E-5*** | 3.471E-5*** |
| Daily protein intake (g/d) | 0.001*** | 0.001*** |
| Serum 25(OH)D (ng/mL) | 0.001 | 0.000 |
Table 3.
|
BMD by quartiles of daily sodium intake‡ |
BMD by quartiles of daily potassium intake‡ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-value for trend | Q1 | Q2 | Q3 | Q4 | P-value for trend | |
| Total femur | 0.000 | 0.006 (-0.012 to 0.025) | 0.003 (-0.015 to 0.021) | -0.012 (-0.034 to 0.009) | 0.226 | 0.000 | -0.004 (-0.023 to 0.015) | 0.004 (-0.016 to 0.024) | 0.009 (-0.014 to 0.032) | 0.373 |
| Lumbar spine | 0.000 | -0.007 (-0.031 to 0.017) | -0.012 (-0.036 to 0.013) | -0.031 (-0.058 to -0.004) | 0.023 | 0.000 | 0.007 (-0.018 to 0.032) | 0.010 (-0.018 to 0.038) | 0.024 (-0.008 to 0.056) | 0.164 |
Table 4.
|
BMD by quartiles of sodium/potassium‡ |
|||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-value for trend | |
| Total femur | 0.000 | -0.021 (-0.037 to -0.005) | -0.009 (-0.025 to 0.007) | -0.023 (-0.042 to -0.005) | 0.044 |
| Lumbar spine | 0.000 | -0.020 (-0.041 to 0.002) | -0.027 (-0.046 to -0.008) | -0.033 (-0.055 to -0.012) | 0.002 |
REFERENCES
1. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. Geneva: World Health Organization; 1994.
2. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil 2010;1:6-14.




3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care 2011;17 Suppl 6:S164-9.

4. National Osteoporosis Foundation. Osteoporosis fast facts [Internet]. Washington (DC): National Osteoporosis Foundation; 2015 [cited 2019 Feb 14]. Available from: https://www.bonehealthandosteoporosis.org/wp-content/uploads/2015/12/Osteoporosis-Fast-Facts.pdf
5. Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res 1997;12:24-35.


6. Delaney MF. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am J Obstet Gynecol 2006;194(2 Suppl):S12-23.


7. Wlodarek D, Glabska D, Kolota A, Adamczyk P, Czekajlo A, Grzeszczak W, et al. Calcium intake and osteoporosis: the influence of calcium intake from dairy products on hip bone mineral density and fracture incidence: a population-based study in women over 55 years of age. Public Health Nutr 2014;17:383-9.


8. Park SM, Joung JY, Cho YY, Sohn SY, Hur KY, Kim JH, et al. Effect of high dietary sodium on bone turnover markers and urinary calcium excretion in Korean postmenopausal women with low bone mass. Eur J Clin Nutr 2015;69:361-6.



9. Kim Y, Kim HY, Kim JH. Associations between reported dietary sodium intake and osteoporosis in Korean postmenopausal women: the 2008-2011 Korea National Health and Nutrition Examination Survey. Asia Pac J Public Health 2017;29:430-9.



10. Park Y, Kwon SJ, Ha YC. Association between urinary sodium excretion and bone health in male and female adults. Ann Nutr Metab 2016;68:189-96.



11. Jones G, Beard T, Parameswaran V, Greenaway T, von Witt R. A population-based study of the relationship between salt intake, bone resorption and bone mass. Eur J Clin Nutr 1997;51:561-5.



12. Fatahi S, Namazi N, Larijani B, Azadbakht L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta-analysis. J Am Coll Nutr 2018;37:522-32.


13. Zhu K, Devine A, Prince RL. The effects of high potassium consumption on bone mineral density in a prospective cohort study of elderly postmenopausal women. Osteoporos Int 2009;20:335-40.



14. Bedford JL, Barr SI. Higher urinary sodium, a proxy for intake, is associated with increased calcium excretion and lower hip bone density in healthy young women with lower calcium intakes. Nutrients 2011;3:951-61.



15. Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res 2013;28:497-504.


16. Cao WT, He J, Chen GD, Wang C, Qiu R, Chen YM. The association between urinary sodium to potassium ratio and bone density in middleaged Chinese adults. Osteoporos Int 2017;28:1077-86.



17. Korea Health Industry Development Institute. National food & nutrition statistics 2011: based on 2011 Korea National Health and Nutrition Examination Survey. Cheongju: Korea Health Industry Development Institute; 2013.
18. Hallstrom H, Byberg L, Glynn A, Lemming EW, Wolk A, Michaelsson K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol 2013;178:898-909.


19. Fung TT, Arasaratnam MH, Grodstein F, Katz JN, Rosner B, Willett WC, et al. Soda consumption and risk of hip fractures in postmenopausal women in the Nurses’ Health Study. Am J Clin Nutr 2014;100:953-8.



20. Okada E, Okada C, Matsumoto M, Fujiwara A, Takimoto H. Dietary sodium:potassium ratio and CVD risk factors among Japanese adults: a retrospective cross-sectional study of pooled data from the National Health and Nutrition Survey, 2003-2017. Br J Nutr 2021;125:79-91.


21. Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open 2016;6:e011632.



22. Mirmiran P, Bahadoran Z, Nazeri P, Azizi F. Dietary sodium to potassium ratio and the incidence of hypertension and cardiovascular disease: a population-based longitudinal study. Clin Exp Hypertens 2018;40:772-9.


23. Willey J, Gardener H, Cespedes S, Cheung YK, Sacco RL, Elkind MS. Dietary sodium to potassium ratio and risk of stroke in a multiethnic urban population: the Northern Manhattan Study. Stroke 2017;48:2979-83.



24. Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention followup study. Arch Intern Med 2009;169:32-40.



25. Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 2007;356:1966-78.


26. Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 2003;133:3130-6.


27. Blackwood AM, Sagnella GA, Cook DG, Cappuccio FP. Urinary calcium excretion, sodium intake and blood pressure in a multi-ethnic population: results of the Wandsworth Heart and Stroke Study. J Hum Hypertens 2001;15:229-37.



28. Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 1998;27:465-83.


29. Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2013;98:207-17.


30. Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, et al. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr 2008;88:465-74.









