 |
 |
- Search
| Korean J Fam Med > Volume 44(1); 2023 > Article |
|
Abstract
Background
Erythropoietin (EPO), which is associated with anemia, exerts neuroprotective effects in ischemic stroke. In cases of stenosis or narrowing of the main cerebral blood vessel, the prognosis is favorable if collateral blood circulation is well developed in acute stroke. Several studies have investigated the relationship between EPO administration and stroke outcomes. The present study investigated the correlation between serum EPO level and cerebral collateral circulation, which could result in favorable clinical outcomes.
Methods
The study subjects were patients diagnosed with acute ischemic stroke who underwent initial brain magnetic resonance imaging between January 2020 and March 2022. Following brain computed tomography perfusion for collateral flow, serum EPO levels were measured. Collaterals were assessed according to the Mass system and divided into good collateral (GC) or poor collateral (PC) groups. Serum EPO levels were determined using a chemiluminescence immunoassay method. A correlation coefficient analysis was conducted to determine the correlation between serum EPO levels and GC. A receiver operating characteristic curve analysis determined the cutoff value of EPO for GC.
The clinical outcome of patients with acute ischemic stroke is poor if the serum erythropoietin (EPO) level is high [1]. However, transient cerebral ischemia or small infarction occurred in patients with previously known severe stenosis, leading to better outcomes. This phenomenon, called ŌĆ£ischemic preconditioning,ŌĆØ involves EPO [2]. Although the previous results may seem contradictory, EPO level increases in the condition of acute stroke to alleviate symptom worsening. In patients with previously known intra- and extracranial stenosis, preventatively elevated EPO suppresses brain cell death and can alleviate symptom worsening. The collateral cerebral circulation affects stroke prognosis. Despite stenosis or narrowing of the main cerebral blood vessels, the prognosis is favorable if the collateral blood circulation is well developed [3]. In this context, EPO and cerebral collateral circulation could be correlated. To date, no study has examined whether the degree of collateral circulation development is correlated with EPO in the acute stage of stroke. The present study investigated for the first time whether there is a correlation between cerebral collateral circulation degree and EPO level.
The subjects of the study were patients diagnosed with acute ischemic stroke by initial brain diffusion-weighted magnetic resonance imaging between January 2020 and March 2022. The inclusion criteria were Korean race, age 18ŌĆō80 years, no acute hemorrhage on initial brain non-contrast computed tomography (CT), definite neurologic deficit defined as a National Institute of Health Stroke Scale (NIHSS) Ōēź5, and onset (clear onset or time from last normal) within 24 hours. The exclusion criteria were as follows: past cerebral hemorrhage (intracranial hemorrhage, subarachnoid hemorrhage), reported atrial fibrillation, presenting seizure activity with acute stroke, and symptomatic regression of neurologic deficit in 1 hour. This study was approved by the Institutional Review board of Public Institutional Review Board Designated by Ministry of Health and Welfare (P01-202106-11-001). Informed consent was obtained from all individual participants included in the study.
In patients with no contraindications for the radio-contrast injection (specifically, contrast allergy or serum creatinine levels >1.5 mg/dL), high-resolution CT angiography (CTA) was performed on a 512-section multidetector helical scanner (Aquilion One; Toshiba Medical Systems, Otawara, Japan), and images were acquired with a 70-mL bolus injection of contrast at 3 mL/s followed by 30 mL of saline at 3 mL/s into the antecubital vein. Scanning was triggered using bolus tracking, with the region of interest placed in the posterior aortic arch and the trigger threshold set at 150 Hounsfield units. The scan parameters at Hongik Hospital were as follows: section thickness, 1 mm; no section gap; field of view, 200 mm; matrix, 512├Ś512; 100ŌĆō120 mAs; and 100 kVp. Coverage extended from the base of the skull to the vertex, and the source images were reformatted into 3-mm-thick axial, coronal, and sagittal projections. Maximum-intensity projections were routinely provided as part of CTA, and no three-dimensional reconstructions were performed. Each CTA study was evaluated for intracranial collaterals according to the Mass system (Figure 1) [4], a 5-point score that compares collaterals on the affected versus unaffected sides. The Mass system uses Sylvian fissure vessels or leptomeningeal collaterals as internal controls. The score ranges were: 5 (exuberant), 4 (more than those on the contralateral side), 3 (equal to those on the contralateral side), 2 (less than those on the contralateral side), and 1 (no vessel opacification). Scores of 3ŌĆō5 were considered good collaterals (GC), while those of 1ŌĆō2 were considered poor collaterals (PC). A brain CT perfusion study was conducted after patient consent was obtained.
Serum EPO levels were obtained from the initial blood sample within 30 minutes of the subject arriving at the emergency room and before any medication was administered. The EPO examination was conducted after patient consent was obtained. Levels were determined using the chemiluminescence immunoassay method.
The modified Rankin score (mRS) system was used for clinical outcome assessment at 3 months after discharge. The mRS is a well-validated, clinician-reported measurement of global disability widely used for evaluating recovery from stroke. Score of 0ŌĆō2 is considered a good outcome and 3ŌĆō5 as a poor outcome (mRS description: 0, no symptoms; 1, able to perform all usual activities despite some symptoms; 2, unable to perform all previous activities; 3, requiring some help but able to walk unassisted; 4, unable to walk unassisted; and 5, bedridden).
FisherŌĆÖs exact test or the chi-square test was used to analyze categorical variables. Differences in continuous variables were evaluated using Student t-test. A binominal logistic regression analysis was performed to evaluate the factors contributing to the GC and PC groups. A correlation coefficient analysis was conducted to determine the correlation between serum EPO levels and GC. A receiver operating characteristic (ROC) curve analysis was conducted to determine the EPO cut-off value for GC. Statistical significance was set at P<0.05, and the analyses were performed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
A total of 56 subjects were enrolled (GC, 22; PC, 34). The basic characteristics of the participants are summarized in Table 1. The frequencies of chronic heart failure (CHF), hospitalization days (HOD), and mRS were higher in the PC group (P<0.05), whereas the frequencies of chronic kidney disease (CKD) and initial EPO were higher in the GC group (P<0.05). Other characteristics such as hemoglobin level, body mass index, prevalence of hypertension, diabetes, dyslipidemia, initial NIHSS score, and Alberta stroke program Early CT Score did not differ significantly between the two groups (P>0.05).
The initial serum EPO level was 85.4% higher (11.5┬▒3.8 mIU/mL versus 6.3┬▒2.1 mIU/mL) in the GC versus PC group. Additionally, the correlation coefficient analysis showed a positive correlation between an elevated serum EPO level and GC after the adjustment for other variables, such as CHF, CKD, HOD, initial NIHSS score, and mRS score (r=0.712, P=0.042). The ROC curve analysis showed that the serum EPO cut-off value for GC was 9.1 mIU/mL with a sensitivity and specificity of 0.79 and 0.86, respectively (area under the curve, 0.888; standard error, 0.044; 95% confidence interval, 0.801ŌĆō0.974; P<0.001) (Figure 2, Table 2).
The present study investigated the correlation between initial serum EPO level and cerebral collateral circulation and analyzed the serum EPO cut-off level (9.1 mIU/mL) for GC. EPO is a peptide produced by the kidneys that increases erythropoiesis by suppressing erythroid precursor apoptosis in the bone marrow to counteract anemia [5]. EPO and its receptors are located in the brain and regulated in response to ischemic stroke [6]. An increase in serum EPO level is associated with better functional outcome in ischemic stroke [7]. Collateral cerebral blood flow in patients with acute ischemic stroke can be evaluated via a CT perfusion study using the collateral index, which showed better clinical outcomes [8].
EPO levels are elevated in coronary chronic total occlusion patients and they had better coronary collateral vessel grade with reduced mortality [9]. On the other hand, there was also conflicting result suggesting that initial elevated EPO level is associated with poor outcome in ischemic stroke [1]. The authors suggested that EPO is mainly a direct indicator of previous anemia, which is a known negative factor for stroke severity and recovery. There is another negative phase II/III clinical study that failed to show positive effects of intravenously administered EPO within 48 hours after stroke onset. However, it should be noted that chronic severe anemia or vessel stenosis significantly stimulates the growth of collaterals [10]. The mean subject age was relatively low. In contrast, no significant correlation was noted between anemia and collaterals in the present study. However, our findings suggest that the elevated serum EPO levels in patients with acute cerebral infarction who visited the emergency department is probably related to the previous development of the cerebral collateral circulation. This is because a previous prospective study revealed that serum EPO concentration continues to increase during the acute stroke period to recover functional outcome [11]. Therefore, our data for elevated serum EPO from the initial acute stroke period implicates previously programmed protective mechanisms from brain injury, such as collateral flow.
A normal EPO level allows sufficient erythrocytes to replace dead cells in the blood and maintain oxygen flow to the renal oxygen sensor to ensure baseline levels of EPO synthesis [12]. The red blood cell production and mass are regulated by EPO and reduced oxygen in arterial blood due to anemia results in increased EPO production [13]. EPO also acts as an angiogenic factor and regulates angiogenesis [14]. Endothelial cell migration and proliferation are the key features in the angiogenic process and are stimulated by recombinant human EPO [15]. Another benefit of a high serum EPO level involves its neuroprotective feature and promotion of the regeneration of injured neurons after stroke [16]. However, in the acute stage of ischemic stroke, GC circulation is the most important finding that may lead to successful reperfusion in ischemic stroke and improve stroke outcome with thrombolytic and endovascular therapy [17]. Therefore, an initial high serum EPO concentration in acute ischemic stroke patients could be a good predictor of GC, leading to a good prognosis. Due to this advantage, it is clinically meaningful that the present study determined an accurate serum EPO cut-off value (9.1 mIU/mL) for GC to provide standards that can be referenced in emergency stroke treatment.
Figure.┬Ā1.
Mass system. (A) No vessel opacification (arrow). (B) Less opacification than on the contralateral side (arrow). Opacification equal to that on the contralateral side is not shown. (C) More opacification than on the contralateral side (arrow). (D) Exuberant opacification is shown (arrow).
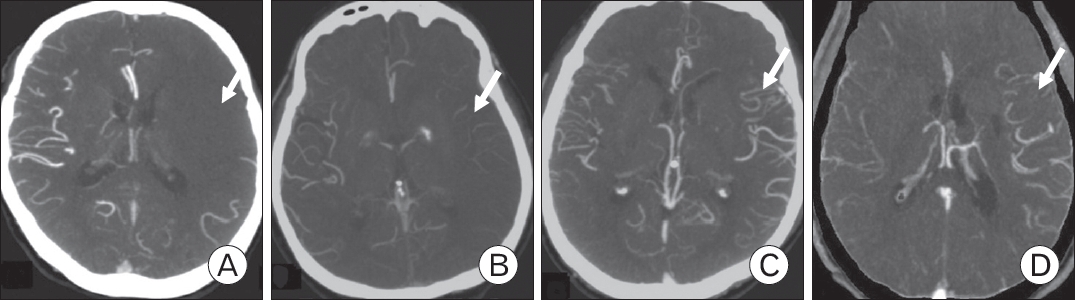
Figure.┬Ā2.
Receiver operating characteristic (ROC) curve of serum erythropoietin and a good collateral (GC). High serum erythropoietin levels showed a positive correlation with GC after that adjustment for other variables such as chronic heart failure, National Institute of Health Stroke Scale, chronic kidney disease, Alberta Stroke Program Early Computed Tomography Score, and hospitalization day (r=0.712, P=0.04).

Table┬Ā1.
Basic characteristics in subjects
REFERENCES
1. Aberg ND, Stanne TM, Jood K, Schioler L, Blomstrand C, Andreasson U, et al. Serum erythropoietin and outcome after ischaemic stroke: a prospective study. BMJ Open 2016;6:e009827.



2. Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res 2006;83:19-27.


3. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab 2011;31:1293-301.



4. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009;40:3001-5.



5. Noguchi CT, Wang L, Rogers HM, Teng R, Jia Y. Survival and proliferative roles of erythropoietin beyond the erythroid lineage. Expert Rev Mol Med 2008;10:e36.



6. Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol 2001;101:271-6.



7. Yip HK, Tsai TH, Lin HS, Chen SF, Sun CK, Leu S, et al. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care 2011;15:R40.



8. Lin L, Chen C, Tian H, Bivard A, Spratt N, Levi CR, et al. Perfusion computed tomography accurately quantifies collateral flow after acute ischemic stroke. Stroke 2020;51:1006-9.


9. Yuksel IO, Cagirci G, Koklu E, Yilmaz A, Kucukseymen S, Ellidag HY, et al. Erythropoietin stimulates the coronary collateral development in patients with coronary chronic total occlusion. Neth Heart J 2016;24:609-16.




11. Ma Y, Zhou Z, Yang GY, Ding J, Wang X. The effect of erythropoietin and its derivatives on ischemic stroke therapy: a comprehensive review. Front Pharmacol 2022;13:743926.



12. Schuster SJ, Caro J. Erythropoietin: physiologic basis for clinical applications. Vox Sang 1993;65:169-79.


13. Ribatti D, Vacca A, Roccaro AM, Crivellato E, Presta M. Erythropoietin as an angiogenic factor. Eur J Clin Invest 2003;33:891-6.



14. Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int 1995;47:740-5.


15. Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004;35:1732-7.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,895 View
- 54 Download
- Related articles in KJFM
-
Relation between Health Habits and Stree in Adolescents.1999 October;20(10)






