 |
 |
- Search
| Korean J Fam Med > Volume 45(1); 2024 > Article |
|
Abstract
Background
Alternative and complementary medicines are widely used to treat migraine headaches. This review aimed to determine the effectiveness of essential oils as an alternative treatment approach.
Methods
A structured search was conducted to identify randomized trials comparing essential oils with a placebo for migraine headaches, using databases (MEDLINE and CENTRAL) to search for articles published between 1966 and 2021. We included trials involving adult males and females diagnosed with migraine headaches according to the International Headache Society. The outcomes included number of attacks, headache severity, associated symptoms, number of days of limited activity, headache duration, use of analgesics, and adverse effects. Seven trials were included with a total of 558 participants.
Results
No difference was observed in the number of migraine headache attacks compared to placebo (mean difference [MD], -1.34; 95% confidence interval [CI], -3.31 to 0.64; I2=94%; P=0.190; four trials, 242 participants; moderate- quality evidence). There was no difference in this outcome between the essential oils treated group and the placebo (MD, -0.38; 95% CI, -1.76 to 0.99; I2 statistics=86%; P=0.580; five trials, 240 participants; moderate-quality evidence).
Migraine is a severe and complex neurovascular disease that affects 16% of the population. It starts in childhood and increases between the ages of 22 and 55 years, affecting females more than males (3:1) with a family history of migraine [1]. It is a chronic condition with occasional symptoms and is ranked as the sixth most disabling disease in the world and the most disabling neurological disorder by the World Health Organization. Financially, it has a significant impact on economies globally which costs US$19.6 billion annually [1].
Migraine is characterized by pulsatile pain on one or both sides of the head as well as other symptoms such as photophobia, phonophobia, nausea, and vomiting [2]. According to the International Classification of Headache Disorders, third edition, a migraine should comprise at least five attacks over the course of a lifetime, last 4–72 hours, and have at least two of the following characteristics: unilateral location, pulsating/throbbing quality, moderate to severe intensity, aggravation by/avoidance of routine physical activity, nausea and/or vomiting, photophobia, and phonophobia [3].
Despite the use of pharmacological medications for migraine headache treatment, the use of non-pharmacological therapy to alleviate migraine headache symptoms is growing and underestimated [4]. These include neuromodulators, acupuncture, behavioral therapies, relaxation training, avoidance of food triggers, and adequate sleep and exercise. According to a major population study, many patients with migraines use complementary and alternative medicines to relieve symptoms [5]. Lavender, peppermint, chamomile, anise, basil, rose, and mixed essential oils have been found to reduce migraine intensity and frequency. Some oils can also reduce the symptoms of photophobia, phonophobia, nausea, and vomiting [2].
A systematic review of the alternative therapies used in headache treatment was published by Lopresti et al. [6]; however, no meta-analysis was performed. This review includes all types of herbal treatments used for migraine headaches, including essential oils. Four of the 19 trials evaluated the effect of essential oils on migraine. They reported that herbal medicine, via its multifactorial physiological influence, is a potential option for enhancing the treatment of migraine [6].
Thus, there is a need to conduct a meta-analysis of alternative therapies for migraine. The findings of this systematic review and meta-analysis will be helpful for healthcare personnel in deciding on and providing the best care for patients with migraines. As alternative medicines are widely used by patients with migraine, the effectiveness of this treatment should be based on evidence, and healthcare personnel should be well-versed in it.
This meta-analysis was conducted to evaluate the effectiveness of essential oils as alternative treatments for migraine in adults. These findings are expected to help healthcare personnel make decisions when treating patients with migraine.
The protocol for this meta-analysis was registered in the International Prospective Register of Systematic Review (PROSPERO) with trial number CRD42022306382 and is available at https://www.crd.york.ac.uk/prospero. This review was performed in accordance with the Cochrane Systematic Review guidelines and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, and Epistemonikos databases for articles published from 1966 to December 2021. We used the search terms “essential oils,” “aromatherapy,” “alternative medicine,” “complementary medicine,” “natural medicine,” AND and OR “migraine,” with Boolean operators AND and OR. We checked the reference list to identify randomized controlled trials (RCTs) and review articles to identify unpublished trials or trials that were unidentified by electronic searches. We searched for ongoing trials using the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov.
We included only RCTs comparing essential oils with placebo or as an adjunct treatment. Non-randomized clinical trials were excluded. Participants were adults aged 18–65 years, of either sex or ethnicity, and diagnosed with migraine according to the International Headache Society criteria by a neurologist or physician. Trials published in nonEnglish languages (one trial in Iran) were excluded because we lacked language resources (professional translators). The intervention included essential oil supplementation regardless of the treatment duration.
We screened the titles and abstracts from the search and obtained fulltext articles when they met the eligibility criteria or when there was insufficient information to assess their eligibility. We assessed the eligibility of the trials independently and documented all reasons for exclusion. Disagreements between the authors were resolved through discussion. Any disagreements were resolved by consensus. The authors were contacted if further clarification was required.
We extracted data on the type of essential oils used, criteria for diagnosis of migraine, age, sex, ethnicity, and the outcome of each trial, including the number of headache attacks, headache severity, headache-associated symptoms such as photophobia, phonophobia, nausea, vomiting, number of days lost/limit activities, duration of headache, use of analgesics and adverse effect of using essential oils, using the data extraction form.
The primary outcomes included the number of attacks, headache severity, and headache-associated symptoms such as photophobia, phonophobia, nausea, and vomiting. The secondary outcomes were the number of days of lost/limited activities, duration of headache, use of analgesics, and adverse effects, such as skin redness and hypersensitivity.
The Cochrane Collaboration tool was used to assess the risk of bias. We assessed the selection bias (randomization and allocation concealment), performance bias (blinding of participants and health personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other types of bias (recall bias, transfer bias, etc.) [7]. We assessed the quality of evidence for primary and secondary outcomes according to the Grading Recommendation Assessment, Development, and Evaluation (GRADE) methodology for the risk of bias, inconsistency, indirectness, imprecision, and publication bias, classified as very low, low, moderate, or high [8].
We analyzed the data using Review Manager ver. 5.4 software (Cochrane, London, UK) [9]. A random-effects model was used to pool the data. Heterogeneity was assessed in two steps. First, we assessed the obvious heterogeneity in face values by comparing populations, settings, interventions, and outcomes. Next, we assessed the statistical heterogeneity using the I2 statistic [7]. We interpreted the heterogeneity as 0% to 40% to represent “might not be important,” 30% to 60% to represent “moderate heterogeneity,” 50% to 90% to represent “substantial heterogeneity,” and 75% to 100% to be “considerable heterogeneity.” [7] We measured the treatment effect using the risk ratio and risk difference for dichotomous outcomes and mean differences (MDs) or standardized MD, both with 95% confidence intervals (CIs) for continuous outcomes. Subgroup analyses were not performed due to the limited number of trials. We explored the potential sources of heterogeneity when available. We performed a sensitivity analysis to investigate the impact of the risk of bias on sequence generation and allocation concealment of the included studies.
A total of 2,200 citations were identified using the original and two updated searches. Following deduplication and inclusion of any additional records, 47 citations were retrieved. Of these, 44 were judged as potentially eligible based on their titles and abstracts. After obtaining full-text publications and assessing eligibility, seven trials were included and 37 were excluded (Figure 1).
We included seven studies with a total of 558 participants. Five of the seven studies received university funding. All the seven trials were conducted in Iran. Four trials were conducted in clinics, two in universities, and one in a hospital. All participants were randomized into intervention and control groups.
The trials involved lavender oil [10,11], anise oil [12], basil oil [13], rose oil [14], peppermint oil [15], and chamomile oil [16]. Four trials topically applied essential oils topically [12-14,16], one involved oral consumption [10], one involved intranasal administration [15], and one involved inhalation [11]. Four of seven trials tested liquid paraffin as a placebo [11,13,14,16], one used a hydroalcoholic solvent [10], one used a cold cream base as a placebo [12], and one did not describe the composition of the placebo [15].
ive trials excluded pregnant participants. Four trials also excluded participants who developed side effects due to the oil. Two trials excluded participants who had undergone other prophylactic treatments. Table 1 summarizes the characteristics of the included studies.
Seven trials were included in the analysis of the primary outcomes. Secondary outcomes were reported in six trials. One trial reported using questionnaires to assess the quality of life affected by migraines, which was the Headache Impact Test-6 [12]. This questionnaire consists of six questions with five answers each: always, very often, sometimes, rarely, and never. The total score ranged from 36 to 78, with a higher score representing a more negative impact of headaches on the quality of life. Another trial adopted the Migraine Disability Assessment questionnaire to assess disability caused by migraines [10]. A score between 0 and 10 represents the severity of the headache. Three studies used the Visual Analog Scale to measure pain intensity. The score ranged from 0 (no pain) to 10 (the most severe pain) [11,13,14].
Four trials described the randomization method used with a low risk of bias. Two trials used the randomization block method [10,12], one used the permutation block method [13], and one used a computer-generated non-stratified block randomization list [14]. The method of randomization was not reported in the other three trials; thus, we judged random sequence generation as having an unclear risk of bias [11,15,16]. Allocation concealment was judged as an unclear risk in two trials because details of the drugs and placebo used were not reported [11,15].
Five trials mentioned the blinding of participants and personnel [10,12-14,16] and were considered low risk. Health personnel in one trial were not blinded; therefore, the risk of bias was high [11]. Blinding of participants and personnel was not mentioned in one trial; therefore, the risks were unclear [15].
Three trials did not mention blinding of the outcome assessor [11,15,16] and thus were judged as having unclear risks. The remaining four trials had a low risk of bias, as the details of the blinding have been described [10,12-14].
Three trials reported a high risk, as they had more than 20% loss to follow-up, with no intention to treat [12,14,16]. One trial had an unclear risk, as loss of data was not mentioned [11]. Three trials were judged as low risk [10,13,15]. No other potential source of bias was identified. The risk of bias graph and summary are presented in Figure 2 and Figure 3, respectively.
Two trials represented crossover studies with a 1-week [14] and 2-week [16] washout periods. Both trials involved observing participants’ responses for 24 hours. In the following trials, participants’ responses were observed for over 30 minutes [15], 2 hours [11], 6 weeks [12], 3 months [10], and 12 weeks each [13].
There was no difference in the number of migraine headache attacks among the four trials (MD, -1.34; 95% CI, -3.31 to 0.64; I2=94%; P=0.190; four trials, 242 participants; moderate-quality evidence) (Figure 4, Table 2) [10,12,15,16]. There was a difference in the effect estimate when data from one trial were excluded [15]. Subgroup analysis for the types of essential oils and administration was not feasible because of the limited number of trials. One trial reported no differences between groups; however, the data were not in a usable format [13].
All seven trials reported the severity of the migraine headaches. Five trials showed no difference in this outcome between the essential oils and placebo group (MD, -0.38; 95% CI, -1.76 to 0.99; I2 statistics=86%; P=0.580; five trials, 240 participants; moderate-quality evidence) (Figure 5, Table 2) [10-12,14,16]. Subgroup analysis of the type of essential oil and the type of administration was not feasible owing to the limited number of trials. There was a difference in the effect estimate when the data from one trial were excluded. Sensitivity analysis was performed for unclear bias of allocation concealment because details of the preparation of these drug samples were not discussed. Sasannejad et al. [11] showed a change in the cumulative effect estimate (MD, -1.07; 95% CI, -2.13 to -0.00; I2 statistics=71%; P=0.020; 193 participants). One trial reported no differences in headache severity; however, the data were not in an usable format [13]. The essential oils reduced the number of events for headache severity compared to the placebo but was limited to only one trial (MD, 0.59; 95% CI, 0.45 to 0.78; 78 participants; very low-quality evidence) [15].
Essential oils reduced photophobia compared to the placebo in two trials (MD, 0.41; 95% CI, 0.16 to 1.06; I2 statistics=79%; P=0.070; two trials, 119 participants; low-quality evidence) (Table 2) [11,16]. One trial reported no difference in the mean occurrence of photophobia (MD, -0.24; 95% CI, -2.53 to 2.05; 29 participants; very low-quality evidence) [14].
Essential oils reduced phonophobia in two trials compared to the placebo (MD, 0.45; 95% CI, 0.21 to 0.97; I2 statistics=53%; P=0.150; two trials, 119 participants, low-quality evidence) (Table 2) [11,16]. One trial reported no difference in the mean occurrence of phonophobia (MD, -0.60; 95% CI, -2.99 to 1.79; 29 participants; very low-quality evidence) [14].
Essential oils showed no difference in nausea and/or vomiting compared to the placebo (MD, 0.32; 95% CI, 0.06 to 1.58; I2 statistics=85%; P=0.160; two trials, 119 participants; low-quality evidence) (Table 2) [11,16]. One trial reported no difference in the mean occurrence of nausea and/or vomiting (MD, -0.50; 95% CI, -2.81 to 1.81; P=0.670; one trial, 29 participants; very low-quality evidence) [14].
Essential oils reduced the number of days of lost or limited activities due to migraine headaches compared to the placebo (MD, -10.17; 95% CI, -13.05 to -7.29; I2 statistics=0%; P=0.560; two trials, 92 participants; moderate-quality evidence) (Table 2) [10,12]. One trial reported no difference in the mean number of days lost or limited activities (MD, 0.08; 95% CI, 0.00 to 1.39; P=0.080; one trial, 78 participants; very low-quality evidence) [15].
One trial reported no difference in the duration of the migraine headache episode (MD, -5.23; 95% CI, -11.27 to 0.81; P=0.090; one trial, 37 participants; low-quality evidence) between the essential oils and placebo groups [12]. Essential oils reduced the duration of the headache episode compared to the placebo but were limited to only one trial (MD, 0.58; 95% CI, 0.43 to 0.78; P=0.001; one trial, 78 participants; very low-quality evidence) [15].
One trial reported no difference in the use of analgesics between the essential oils and placebo groups (MD, -0.48; 95% CI, -2.64 to 1.68; P=0.660; one trial, 37 participants; very low-quality evidence) [12].
One event of skin redness was reported after intervention in one trial (MD, 3.20; 95% CI, 0.14 to 72.62; P=0.470; one trial, 29 participants; very low-quality evidence) [14]. One event of hypersensitivity was reported in one trial after intervention (MD, 4.49; 95% CI, 0.22 to 90.30; P=0.330; one trial, 72 participants; very low-quality evidence) [16].
This review included all RCTs that addressed the effectiveness of essential oils as an alternative treatment for migraine in adults. The seven identified trials formed a heterogeneous group that addressed comparisons and various outcomes, resulting in a few trials contributing to each of our predefined outcomes. This study showed that the mean number of migraine headache attacks using essential oils did not differ from that using a placebo. The severity of headache showed no improvements when using essential oils compared to the placebo groups. Participants taking essential oils showed no differences in alleviation of headache-associated symptoms, such as photophobia, compared to the placebo group. The participants showed reduced headache-associated symptoms, such as phonophobia, nausea, and vomiting, compared to the placebo group; however, the results were limited to a small number of trials.
We performed a comprehensive literature review to assess the effectiveness of essential oils as alternative treatments for migraine in adults. The control group received a placebo containing liquid paraffin or a cold cream base. The duration, doses, types, and routes of administration of the essential oils differed in each trial, thereby limiting the applicability of the findings in this review. We could not perform a subgroup analysis because of the limited number of trials available. Based on the reported incidence of adverse events, we were able to detect common side effects such as skin redness and hypersensitivity; however, there is a lack of information on rare and serious adverse events.
The quality of trial evidence varied. Generally, the outcomes ranged from low to moderate risk or bias. Although there were unclear and high risks of bias in some risk bias assessments, we believe that these risks were not significant for the review because headache itself is a subjective assessment, and the outcome would not have affected whether health personnel or participants were unblinded. No evidence of a selective reporting bias was observed. The lack of adequate random sequence generation can lead to a treatment effect bias in the original study and subsequent reviews. The risk of attrition bias was observed in three trials. Attrition bias was unclear in one trial due to unexplained reasons for loss to follow-up. The risk of attrition bias was high in two trials due to >20% loss to follow-up in five trials, and an intention-to-treat analysis was not performed. Five of the trials received funding from universities. We encountered high heterogeneity in the trials reporting responses to the treatment and the occurrence of adverse events, which was probably due to differences in the duration of treatment or the types of essential oils used. Some outcomes showed substantial heterogeneity. However, we could not perform subgroup analyses because of the limited number of trials. Therefore, the overall level of evidence contributed to this review, as assessed using the GRADE approach, was of low-to-moderate quality (Table 2).
We attempted to reduce publication bias by checking the reference lists of all related studies and by searching multiple databases without language restrictions. To reduce publication bias, we checked the protocols and consistency between the objectives, methodology, and results of each trial. Seven studies were included, and we were unable to construct a funnel plot to detect publication bias. Not all the included studies reported all outcomes. We did not perform a meta-regression analysis to assess publication bias.
To date, only one systematic review has been published on this topic [6]. However, no meta-analyses have yet been performed. One systematic review, without a meta-analysis, included all types of herbal treatments used for migraine headaches, including essential oils. Four of the 19 trials evaluated the effects of essential oils on migraine. They reported that herbal medicines, owing to their multifactorial physiological influences, present a potential option for enhancing migraine treatment of migraine [6]. We found no other systematic reviews that reported other prespecified secondary outcomes. Data on the side effects of essential oils are limited and merit further investigation. Further studies should be conducted using larger sample sizes.
In this review, we found no significant differences in the use of essential oils as alternative treatments for migraine headaches. Data on the rarer and more serious adverse effects of essential oils are limited, and more safety data are needed to better assess whether essential oils are useful interventions as the main treatment for migraine headaches.
Further research is required to determine the efficacy and safety of essential oils as an alternative treatment for migraine headaches in adults. Many previous trials did not mention any serious side effects or rates of withdrawal, and the reporting of adverse events following treatment was not uniform throughout the studies. The low reporting of adverse effects may indicate tolerance and safety. Unfortunately, most studies lacked information that might have masked these factors, and only a small number of participants were included. Thus, it is crucial to obtain reliable tolerability and safety data for all applications of essential oils. Thus, a meta-analysis of the trials will be incomplete because of the lack of data. High-quality trials with large sample sizes that assess quality of life and clinical outcomes are required in future studies.
The primary outcomes were the number of migraine headache attacks and severity of migraine headaches. It is necessary to determine the types of administration and essential oils for the primary outcomes; however, subgroup analysis was not performed because of the limited number of studies. A clinical trial conducted in Iran was also excluded, due to lack of professional and volunteer translators and software translation tools. Further large-scale studies are needed to collect more data on the side effects of essential oils. This may help determine whether essential oils are beneficial as a mainstay treatment for migraine headaches.
Figure. 1.
Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) flow chart of the search results. RCT, randomized controlled trial.
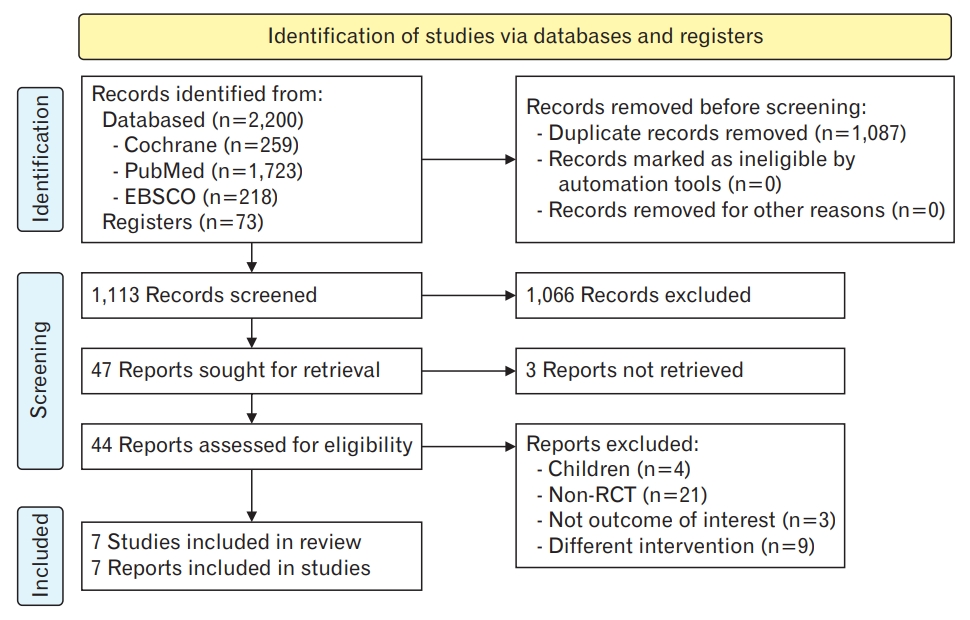
Figure. 4.
Number of attacks. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
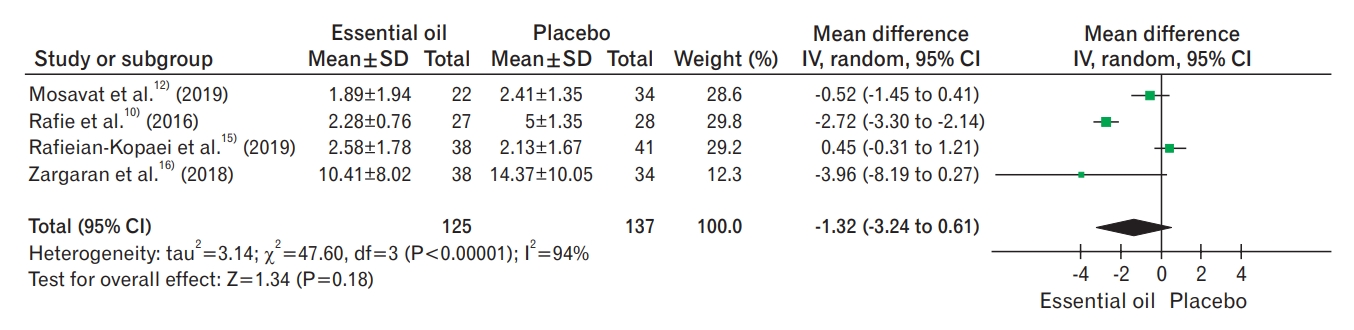
Figure. 5.
Headache severity. SD, standard deviation; IV, inverse variance; CI, confidence interval; df, degrees of freedom.
Table 1.
Characteristics of included studies
| Author (year) | Duration | Participants | Intervention | Control |
|---|---|---|---|---|
| Ahmadifard et al. [13] (2019) | 3 mo | 144 Adults diagnosed with migraine headaches, according to the IHS; 103 females and 38 males; mean age, 34 years (range, 18–46 years) | Basil essential oil 2%, basil essential oil 4%, basil essential oil 6% | Liquid containing pharmaceutical paraffin with a similar appearance, odor to match the basil essential oil |
| Mosavat et al. [12] (2019) | 6 wk | 50 Adults diagnosed with migraine headache according to IHS; mean age 40 years with an age range of 18–65 years; gender distribution not specified | Anise essential oil cream | Cold cream base, packed in a similar jar |
| Niazi et al. [14] (2017) | 2 Consecutive headache | 40 Adults with at least two migraines per month, according to IHS criteria; mean age 35 with an age range of 18–65 years; gender distribution not specified | Rose essential oil | Pharmaceutical paraffin (Merck Co.) with added 0.1% rose essential oil |
| Rafie et al. [10] (2016) | 3 mo | 60 Adults with a long-term history of migraine stacks diagnosed according to IHS criteria for migraine without aura; 18 males, 42 females; age range of 15 to 50 years old | 10 Drops of lavender extract (lavender essential oil dissolved in a hydroalcoholic solvent [ethanol/ water 80/20]) every night | Drops in the same color, same physical form, packaging, and labelling as lavender extract |
| Rafieian-Kopaei et al. [15] (2019) | 2 mo | 120 Adults with a diagnosis of migraine headache, according to IHS criteria; 7 males and 28 females; mean age, 30 years (range, 20–40 years) | 2 Drops of Lidocaine 4% drops/ Peppermint essential oil 1.5% drops intranasally | 2 Drops of placebo intranasally (unsure content) |
| Sasannejad et al. [11] (2012) | 6 Consecutive migraine | 47 Adults with a diagnosis of migraine headache, according to IHS criteria; 34 females and 13 males; mean age 30 years with age, age range not specified | 2–3 Drops of lavender essential oil topically on the upper lip and vapor inhaled for 15 minutes | Liquid paraffin was applied topically on the upper lip and the vapor was inhaled for 15 minutes |
| Zargaran et al. [16] (2018) | 2 Consecutive migraine | 100 Adults with diagnosis of migraine headaches diagnosed according to IHS criteria; 33 males and 87 females, with an age range between 18 to 65 years old | Chamomile oelogel | A mixture of 10% traditional chamomile oil (final product of drug) in liquid paraffin (Merck Co.) |
Table 2.
Summary of the findings, including GRADE quality assessment for comparison between essential oils and migraine headaches
| Outcome |
Certainty assessment |
Summary of findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of the evidence |
Study event rates (%) |
Relative effect (95% CI) |
Anticipated absolute effects |
|||
| With migraine headache | With essential oils | Risk with migraine headache | Risk difference with essential oils | |||||||||
| No. of migraine headache attacks | 242 (4 RCTs) | Not serious | Not serious | Not serious | Serious* | None | ⨁⨁⨁◯ moderate | 121 | 121 | - | The mean number of attacks was 0 | MD 1.34 lower (3.31 lower to 0.64 higher) |
| Headache severity | 240 (5 RCTs) | Not serious | Not serious | Not serious | Serious* | None | ⨁⨁⨁◯ moderate | 115 | 125 | - | The mean headache severity was 0 | MD 0.38 lower (1.76 lower to 0.99 higher) |
| Photophobia | 119 (2 RCTs) | Not serious | Serious† | Not serious | Serious‡ | None | ⨁⨁◯◯ low | 38/57 (66.7%) | 15/62 (24.2%) | RR 0.41 (0.16 to 1.06) | 667 per 1,000 | 393 fewer per 1,000 (from 560 fewer to 40 more) |
| Phonophobia | 119 (2 RCTs) | Not serious | Serious† | Not serious | Serious‡ | None | ⨁⨁◯◯ low | 33/57 (57.9%) | 15/62 (24.2%) | RR 0.45 (0.21 to 0.97) | 579 per 1,000 | 318 fewer per 1,000 (from 457 fewer to 17 fewer) |
| Nausea and/or vomiting | 119 (2 RCTs) | Not serious | Serious† | Not serious | Serious‡ | None | ⨁⨁◯◯ low | 39/57 (68.4%) | 12/62 (19.4%) | RR 0.32 (0.06 to 1.58) | 684 per 1,000 | 465 fewer per 1,000 (from 643 fewer to |
| No. of days lost/limited activities | 92 (2 RCTs) | Not serious | Not serious | Not serious | Serious‡ | None | ⨁⨁⨁◯ moderate | 43 | 49 | - | The mean no. of days lost/limited activities was 0 | MD 10.17 lower (13.05 lower to 7.29 lower) |
REFERENCES
1. Rajapakse T, Davenport WJ. Phytomedicines in the treatment of migraine. CNS Drugs 2019;33:399-415.



2. Yuan R, Zhang D, Yang J, Wu Z, Luo C, Han L, et al. Review of aromatherapy essential oils and their mechanism of action against migraines. J Ethnopharmacol 2021;265:113326.


3. Cutter EE. How to diagnose migraine. Royal (NJ): American Headache Society; 2021.
4. Wells RE, Bertisch SM, Buettner C, Phillips RS, McCarthy EP. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache 2011;51:1087-97.



5. Adams J, Barbery G, Lui CW. Complementary and alternative medicine use for headache and migraine: a critical review of the literature. Headache 2013;53:459-73.


6. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res 2020;34:2493-517.



7. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions, version 6.2 (updated February 2021). London: The Cochrane Collaboration; 2021.
8. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995-8.



9. The Cochrane Collaboration. RevMan: review Manager (RevMan) [Computer program]. Version 5.4. London: The Cochrane Collaboration; 2020.
10. Rafie S, Namjoyan F, Golfakhrabadi F, Yousefbeyk F, Hassanzadeh A. Effect of lavender essential oil as a prophylactic therapy for migraine: a randomized controlled clinical trial. J Herb Med 2016;6:18-23.

11. Sasannejad P, Saeedi M, Shoeibi A, Gorji A, Abbasi M, Foroughipour M. Lavender essential oil in the treatment of migraine headache: a placebo-controlled clinical trial. Eur Neurol 2012;67:288-91.



12. Mosavat SH, Jaberi AR, Sobhani Z, Mosaffa-Jahromi M, Iraji A, Moayedfard A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: a pilot randomized placebo-controlled clinical trial. J Ethnopharmacol 2019;236:155-60.


13. Ahmadifard M, Yarahmadi S, Ardalan A, Ebrahimzadeh F, Bahrami P, Sheikhi E. The efficacy of topical basil essential oil on relieving migraine headaches: a randomized triple-blind study. Complement Med Res 2020;27:310-8.



14. Niazi M, Hashempur MH, Taghizadeh M, Heydari M, Shariat A. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: a randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med 2017;34:35-41.


15. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, Deris F, Solati K, Mahdiyeh F. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med 2019;10:121.



16. Zargaran A, Borhani-Haghighi A, Salehi-Marzijarani M, Faridi P, Daneshamouz S, Azadi A, et al. Evaluation of the effect of topical chamomile (Matricaria chamomilla L.) oleogel as pain relief in migraine without aura: a randomized, double-blind, placebo-controlled, crossover study. Neurol Sci 2018;39:1345-53.












