The Association between Serum Uric Acid and Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus: A Multicenter Nationwide CrossSectional Study
Article information
Abstract
Background
The role of uric acid in the development of diabetic peripheral neuropathy remains unclear. This study aimed to determine the association between uric acid and peripheral neuropathy among type 2 diabetes mellitus (T2DM) patients.
Methods
We conducted a nationwide cross-sectional study based on the diabetes and hypertension study of the Medical Research Network of the Consortium of Thai Medical Schools. Adult T2DM patients from 831 public hospitals in Thailand were evaluated. The serum uric acid level was categorized into five groups based on quintiles (<4.4, 4.4–5.3, 5.3–6.2, 6.2–7.3, and >7.3 mg/dL). A multivariate logistic regression model was used to assess the independent association between serum uric acid level and peripheral neuropathy.
Results
In total, 7,511 T2DM patients with available data about serum uric acid levels were included in the analysis. The mean age of the participants was 61.7±10.9 years, and approximately 35.6% were men. The prevalence rate of peripheral neuropathy was 3.0%. Moreover, the prevalence rates of peripheral neuropathy stratified according to uric acid levels <4.4, 4.4–5.3, 5.3–6.2, 6.2–7.3, and >7.3 mg/dL were 2.5%, 2.8%, 2.4%, 2.5%, and 4.7%, respectively. A serum uric acid level ≥7.3 mg/dL was found to be associated with an increase in odds ratio (1.54; 95% confidence interval, 1.02–2.32) for peripheral neuropathy compared with a serum uric acid level <4.4 mg/dL.
Conclusion
Serum uric acid level is independently associated with peripheral neuropathy in T2DM patients, and elevated serum uric acid levels should be considered a risk factor for diabetic peripheral neuropathy in clinical practice.
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) and its related complications is rapidly increasing worldwide, thereby resulting in significant morbidity and mortality [1]. Diabetic peripheral neuropathy (DPN) is one of the associated complications. This condition can adversely affect quality of life [2] and lead to significant disability and mortality [3]. Furthermore, it is remarkably associated with increased risks of subsequent cardiovascular-related disease and mortality [4]. The factors involved in the pathogenesis of DPN have not been completely understood, and most hypotheses have proposed a multifactorial mechanism [5]. The pathogenesis of DPN may be correlated with various factors, such as the duration of hyperglycemia, dyslipidemia (DLP), obesity, hypertension (HTN), alcohol consumption, smoking, genetic polymorphisms, and plasma homocysteine levels. The molecular etiologic factors may include the polyol pathway, non-enzymatic glycation, free radical, and oxidative stress [6]. The identification of the risk factors associated with DPN may provide further explanations about the development of DPN and may allow advancement of new therapies. Based on previous studies, hyperuricemia is commonly associated with multiple vascular complications [7,8].
Serum uric acid level is widely utilized in assessing the risk of cardiovascular complications. The role of uric acid in the development of vascular complications of diabetes had been assessed [8]. However, its role in the development of DPN remains unclear. Thus, the current study aimed to determine whether there is an association between uric acid and DPN in T2DM patients from a large multicenter nationwide cohort in Thailand. The identification of this relationship may improve the clinical monitoring, early detection, and prevention of DPN.
METHODS
1. Study Design and Population
In 2014, this nationwide, multicenter, cross-sectional study conducted a secondary analysis using a diabetes mellitus and HTN dataset [9]. This dataset was obtained from a nationwide survey conducted annually in Thailand. Then, we evaluated the status of medical care in T2DM patients who visited the public hospitals of the Thai Ministry of Public Health and the clinics included in the Thailand National Health Security Office’s program. The inclusion criteria of this diabetes mellitus and HTN survey were patients aged ≥35 years with HTN who received regular medical care in the targeted hospitals for at least 12 months. Patients who received care at primary care units outside of Bangkok and at university hospitals were excluded from the study. A two-stage stratified cluster sampling method was used to select a nationally and provincially representative sample of patients with T2DM in Thailand. The first stage of sample collection comprised provinces that constituted 77 strata. The second stage of sample collection was composed of hospitals in each province stratified into five strata according to size (Figure 1): regional (>500 beds), provincial (200–500 beds), large community (80–120 beds), medium community (60 beds), and small community (10–30 beds) hospitals. All regional (n=25), provincial (n=70), and community (n=736) hospitals were included. Of 736 community hospitals, 66 (9.0%), 131 (17.8%), and 539 (73.2%) were large, medium, and small community hospitals, respectively. This study aimed to assess the association between serum uric acid level and peripheral neuropathy in T2DM patients. Thus, only patients with available data about baseline serum uric acid levels were included in the analysis.
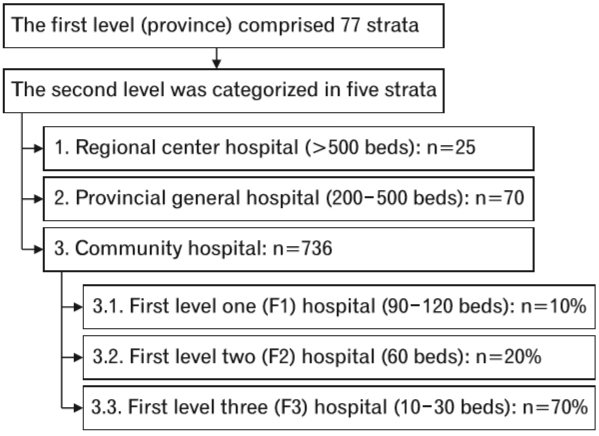
Flowchart of the two-stage stratified cluster sampling method for selecting adult patients with type 2 diabetes mellitus in Thailand.
All patients were recruited from the outpatient clinic. A written informed consent was obtained from the patients before enrolment. Moreover, this study was approved by both the institutional review board of the Royal Thai Army Medical Department and the ethical review committee for Research in Human Subjects, the Ministry of Public Health of Thailand (IRB approval no., s007h/54). Well-trained research nurses reviewed the medical records of the patients and collected data into a case record form. The data in the case record form were then transferred to the central data management of the Medical Research Network of the Consortium of Thai Medical Schools to adjudicate that the process of data collection was performed according to study protocol. The data management team validated the data at the study sites. Site monitoring was randomly performed in approximately 10% of study sites.
2. Data Collection
Data about clinical characteristics, demographic information, medications used, and laboratory examination results were collected via manual data retrieval from the medical record, as described above. The laboratory results were the most recent results within 12 months prior to the consent process. The primary outcome measure was peripheral neuropathy in T2DM patients. The diagnosis of peripheral neuropathy was based on the Neuropathy Disability Score (NDS), which comprises a standardized examination of ankle reflexes, 128-Hz tuning fork sensation, pinprick, and temperature sensation at the hallux. Patients with an NDS score ≥6 were diagnosed with neuropathy [10].
3. Statistical Analysis
Continuous variables were presented as mean±standard deviation and categorical variables as count with percentage. The baseline demographic and clinical characteristics of the serum uric acid groups were compared using analysis of variance for continuous variables and the chi-square test for categorical variables. The serum uric acid level was categorized into five groups based on quintiles (20%, 40%, 60%, and 80%): <4.4, 4.4–5.3, 5.3–6.2, 6.2–7.3, and >7.3 mg/dL. The first lowest quintile group (<4.4 mg/dL) was selected as the reference group for outcome comparison. After adjusting for a priori-defined variables, univariate and multivariate logistic regression analyses were performed to assess the independent association between serum uric acid level and peripheral neuropathy. Odds ratio (OR) with 95% confidence interval (CI) was reported. The adjusted variables were age, gender, duration of T2DM, smoking, comorbidities, medications used, body mass index (BMI), fasting plasma glucose (FPG) level, and estimated glomerular filtration rate (eGFR). The comorbidities were HTN, DLP, coronary artery disease (CAD), cerebrovascular disease (CVD), and diabetic retinopathy (DR). The medications used were insulin, renin-angiotensin-aldosterone system (RAAS) blockers, antiplatelets, and statins. The eGFR was estimated based on age, sex, race, and the most recent creatinine level using the Chronic Kidney Disease Epidemiology Collaboration equation [11]. A pre-specified subgroup analysis based on sex was performed. The serum uric acid level was re-categorized into five groups stratified according to gender based on quintiles (20%, 40%, 60%, and 80%): <5.0, 5.0–5.9, 5.9–6.8, 6.8–7.8, and >7.8 mg/ dL for men and <4.1, 4.1–5.0, 5.0–5.9, 5.9–7.0, and >7.0 mg/dL for women. The first lowest quintile group (<5.0 and <4.1 mg/dL for men and women, respectively) was selected as the reference group for outcome comparison. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS software ver. 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Baseline Characteristics of the Participants
In total, 7,511 adult T2DM patients with available data about serum uric acid levels were included in the analysis. The clinical characteristics of the participants are summarized in Table 1. The mean age of the participants was 61.7±10.9 years, and approximately 35.6% were men. The mean duration of T2DM was 7.4±4.6 years. Each serum uric acid level quintile significantly differed in terms of baseline characteristics, such as age, gender, duration of T2DM, HTN, DLP, CAD, CVD, DR, smoking, use of insulin, RAAS blockade, and antiplatelet drugs, BMI, FPG level, and eGFR (Table 1).
The prevalence rate of peripheral neuropathy was 3.0%. The prevalence rates of peripheral neuropathy stratified according to uric acid levels <4.4, 4.4–5.3, 5.3–6.2, 6.2–7.3, and >7.3 mg/dL were 2.5%, 2.8%, 2.4%, 2.5%, and 4.7%, respectively (P<0.001) (Table 1). Patients with high serum uric acid levels had a significantly increased prevalence of DPN.
2. Association between Serum Uric Acid and Peripheral Neuropathy in Type 2 Diabetes Mellitus Patients after Adjustment
Based on the adjusted model, a serum uric acid level ≥7.3 mg/dL was associated with an increased OR for peripheral neuropathy (OR, 1.54; 95% CI, 1.02–2.32). The serum uric acid levels of 4.4–5.3, 5.3–6.2, and 6.2–7.3 mg/dL were not associated with peripheral neuropathy (Table 2).
3. Subgroup Analyses of the Association between Serum Uric Acid Level and Peripheral Neuropathy in Type 2 Diabetes Mellitus Patients according to Gender
Based on the adjusted model, a serum uric acid level ≥7.0 mg/dL was significantly associated with an increased OR for peripheral neuropathy (OR, 1.68; 95% CI, 1.02–2.78; P=0.04) among female T2DM patients. The subgroup analyses revealed that the statistically significant association was not observed in the male subgroup (P=0.82) (Table 3).
DISCUSSION
This study found that the prevalence rate of peripheral neuropathy was 3.0%, and a high serum uric acid level was independently associated with peripheral neuropathy in T2DM patients, particularly women. In addition, the serum uric acid level group 3 (5.3–6.2 mg/dL) had the lowest prevalence of DPN. This result might be attributed to several causes, that is, the serum uric acid level in this group might be at an optimal level, or there was misclassification due to the limitations of the NDS test [12]. However, our study did not show an inverse association between peripheral neuropathy and this group of serum uric acid levels (5.3–6.2 mg/dL).
The prevalence of peripheral neuropathy in T2DM patients in the current study was comparable to that in previous studies conducted in general outpatient clinics associated with a tertiary center [13] and in a national survey in Thailand [14]. However, the prevalence rate of DPN in the current study was lower than that in other previous studies [15-17]. This result could be explained by the possible inclusion of patients from specialty clinics with more severe comorbidities and associated neuropathy in previous studies. By contrast, our study only included patients from general outpatient clinics with less complicated conditions. Another reason might be the limitations of the NDS test. A previous study found that this test has a sensitivity of 65% and specificity of 91% [12]. Although we do not have available data about nerve conduction study, this test has a high specificity, and it can be easily performed at the bedside.
Currently, the risk factors associated with DPN have not been completely identified, and the pathogenesis of DPN remains unclear. The current study showed that serum uric acid levels ≥7.3 mg/dL are independently associated with peripheral neuropathy in T2DM patients.
Although the pathogenic mechanism for the development of DPN has not yet been fully elucidated, previous studies have shown that inflammatory reaction, oxidative stress, and endothelial dysfunction may contribute to the development of DPN [5,18,19]. Uric acid is widely known as a pro-oxidative and an inflammatory agent. Serum uric acid may be a pro-oxidant via a positive augmentation reaction; uric acid interacts with oxidants to produce more free radicals that promote a radical chain reaction and oxidative damage to cells [20]. Another study showed that after the uptake of uric acid into a cell, it activated mitogen-activated protein kinase, induced cyclooxygenase-2, stimulated the production of local thromboxane, and increased the expression of platelet-derived growth factors A and C and α-receptor mRNA [21]. Moreover, uric acid may contribute to endothelial dysfunction [22]. The results of these studies further support the notion that serum uric acid may play an essential role in the pathogenesis of DPN.
Yu et al. [23] conducted a meta-analysis of 12 small cross-sectional and case-control studies evaluating the association between serum uric acid levels and DPN in T2DM patients. Results showed that hyperuricemia might be associated with an increased risk of peripheral neuropathy in T2DM patients. However, several limitations, including a limited number of eligible studies and their funnel plot, had limited power in detecting the exact risk of publication bias. The authors indicate that more studies with a large number of participants must be conducted [23]. Two subsequent small cross-sectional studies published by Abraham et al. [24] and Lin et al. [25] (a total number of 115 and 200 T2DM patients, respectively) evaluated serum uric acid levels in T2DM patients with and without DPN. In the study of Abraham et al., [24] the serum uric acid levels were correlated with the clinical and electrophysiological severity of diabetic sensorimotor polyneuropathy. Lin et al. [25] showed a significant association between elevated serum uric acid levels and DPN. In addition, results showed that type 2 diabetic patients with sudomotor dysfunction had significantly higher serum uric acid levels [26].
Our study revealed that uric acid has a gender-specific independent effect on DPN. After adjusting for confounders, the association between uric acid and DPN was statistically significant in women but in men. Interestingly, several studies have shown a significantly stronger association between serum uric acid and other diseases, such as atrial fibrillation [27], HTN [28], and renal disease [29], among women. The mechanism for the gender difference is unknown. One hypothesis might be, at least in part, correlated to menopause as estrogen is known to have uricosuric properties [27,28]. Future well-designed studies should be performed to explore the underlying mechanisms.
This study included 7,511 adult T2DM patients from a large nationwide multicenter cross-sectional study. The association was analyzed using the multiple logistic regression model after adjusting for several possible confounders, such as age, gender, duration of T2DM, smoking, comorbidities, medications, BMI, FPG level, and eGFR. The comorbidities were HTN, DLP, CAD, CVD, and DR. Medications were insulin, RAAS blockade use, antiplatelets, and statins for adjusted in the final model.
The current study had several limitations that must be considered. First, the study population does not include patients from university hospitals. Consequently, DPN was not evaluated by neurologists, and this might have led to the non-differential misclassification of DPN, which could result in the over- or under-reporting of the true prevalence of DPN. Second, data collection only included a retrospective medical record review; therefore, missing diagnosis codes or incomplete data could not be obtained. Third, the study only included patients with available data about serum uric acid levels, and this might have caused selection bias as our cohort could be at higher risk for peripheral neuropathy, as medical providers were likely to check this level due to associated medical concerns. Fourth, data about the use of uric-lowering medications were limited, which might have influenced the results of the adjusted model. Fifth, although glucose fluctuation affects the symptoms of DPN [30], data about this factor were not available. Thus, it was not included in the adjusted model. Sixth, the test methods used at each hospital, such as that for creatinine, might differ. Only half of the participating centers used the isotope dilution-mass spectrometry-traceable enzymatic method for the measurement of serum creatinine levels. However, all centers have their own laboratory standard quality control system used in local practice. Last, the current study had a cross-sectional design, and the association of the temporal sequence was limited. Hence, a prospective trial with a similar population must be performed to validate the findings of our study.
Based on our findings, uric acid may be an additional risk marker for DPN. Serum uric acid levels should be monitored for peripheral neuropathy awareness and prevention among T2DM patients.
In conclusion, no changes were observed in the prevalence of peripheral neuropathy in T2DM. Serum uric acid level was independently associated with peripheral neuropathy in T2DM patients, particularly in women. Thus, clinicians should consider serum uric acid levels as another routine monitoring risk marker for peripheral neuropathy in T2DM patients with high serum uric acid levels.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors wish to thank the Medical Research Network of the Consortium of Thai Medical Schools (MedResNet) Thailand, which granted access to the diabetes and HTN dataset in the DAMUS website (http://www.damus.in.th/damus/index.php).



