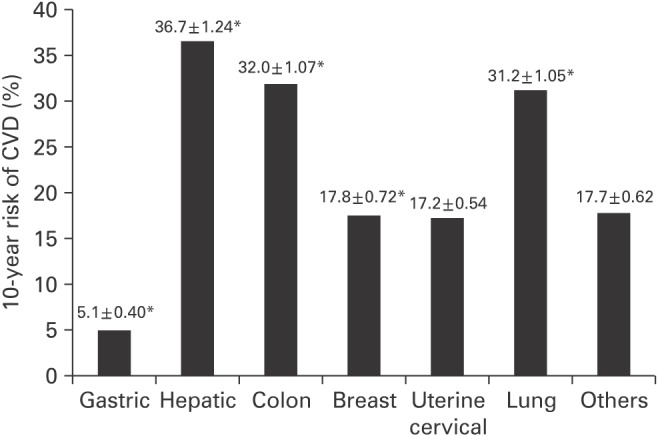
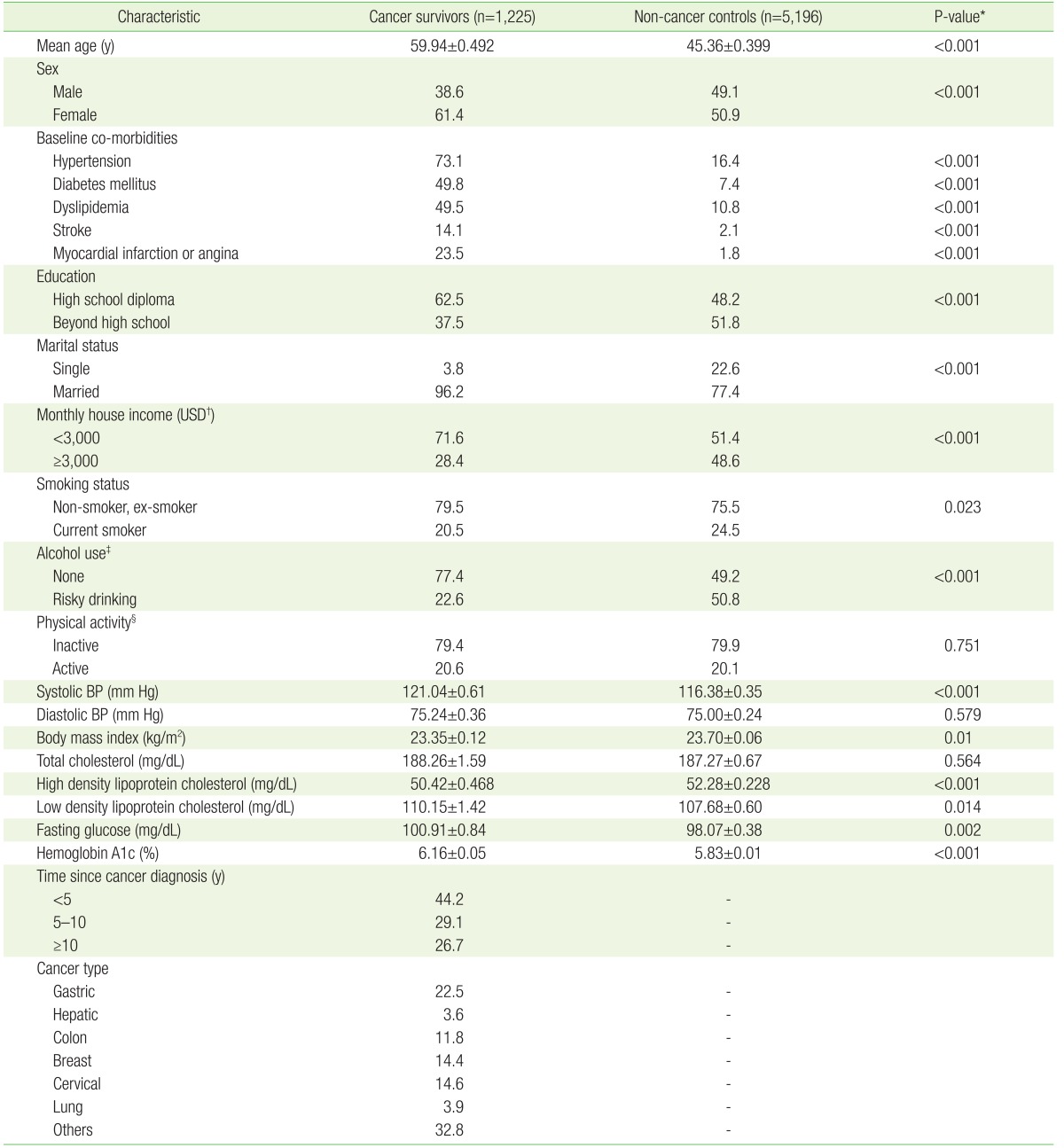
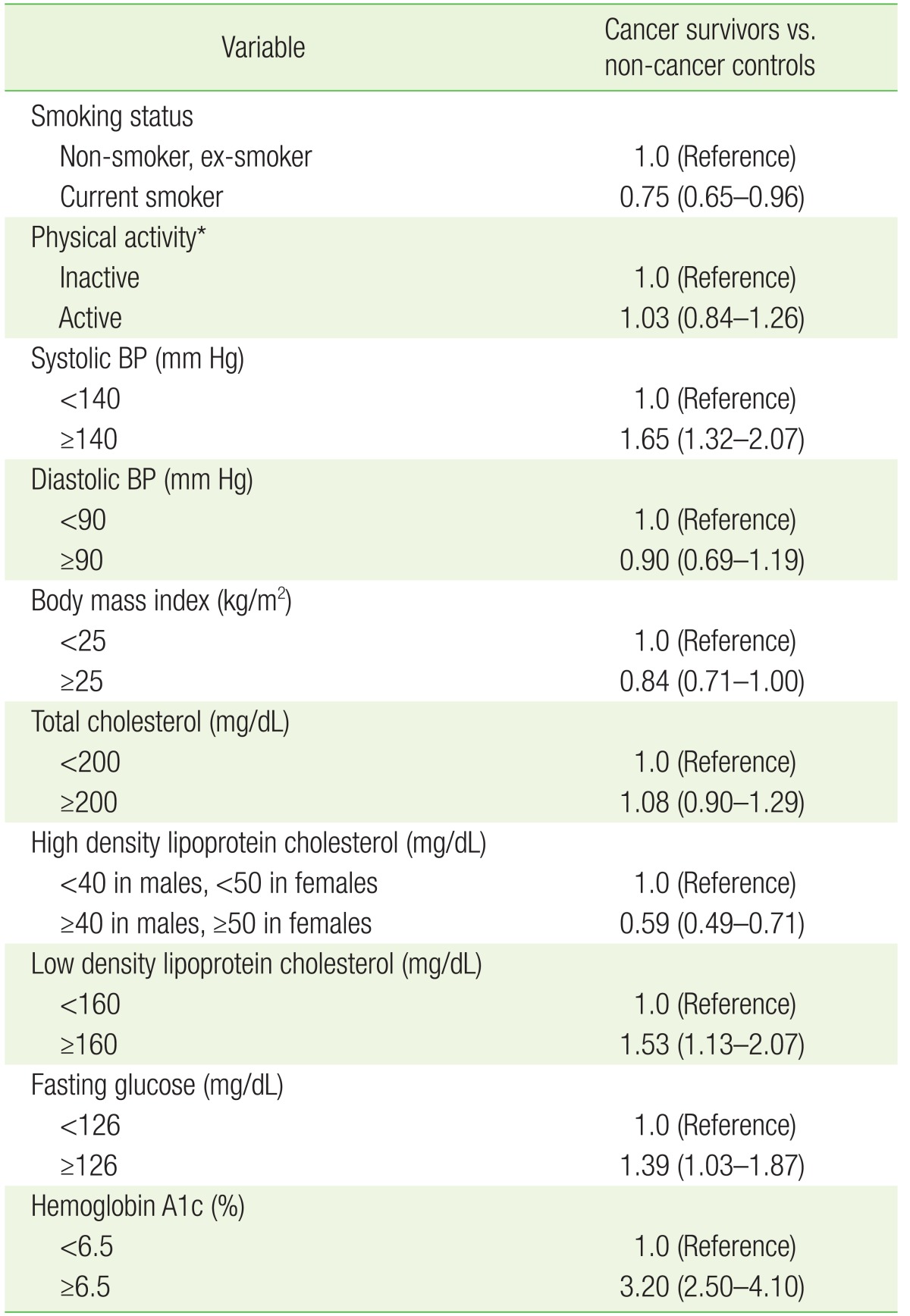
1. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-241. PMID:
22700443.


2. National Cancer Center. Cancer facts and figures. Goyang: National Cancer Center; 2014.
4. Shin DW, Cho B, Kim SY, Jung JH, Park JH. Management of cancer survivors in clinical and public health perspectives: current status and future challenges in Korea. J Korean Med Sci 2013;28:651-657. PMID:
23678254.



5. Baade PD, Fritschi L, Eakin EG. Non-cancer mortality among people diagnosed with cancer (Australia). Cancer Causes Control 2006;17:287-297. PMID:
16489536.


6. Fitzgerald KR. Review of article: the prevention of cardiovascular disease in cancer survivors by Iyad N. Daher, MD, Tina R. Daigle, RN, MSN, Nirmanmoh Bhatia, MD, Jean-Bernard Durand, MD (Tex Heart Inst J 2012;39[2]:190-8). J Vasc Nurs 2012;30:100PMID:
22901450.


7. Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv 2013;7:253-261. PMID:
23417882.



8. Shin DW, Ahn E, Kim H, Park S, Kim YA, Yun YH. Non-cancer mortality among long-term survivors of adult cancer in Korea: national cancer registry study. Cancer Causes Control 2010;21:919-929. PMID:
20169405.


9. Enright KA, Krzyzanowska MK. Control of cardiovascular risk factors among adult cancer survivors: a population-based survey. Cancer Causes Control 2010;21:1867-1874. PMID:
20645125.


10. Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004;109:3122-3131. PMID:
15226229.


11. Wolin KY, Colditz GA. Implementing chronic disease prevention amongst cancer survivors. J Intern Med 2011;269:85-87. PMID:
21054585.


12. Landy DC, Miller TL, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am Heart J 2012;163:295-301.e2. PMID:
22305850.



13. Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol 2005;23:8884-8893. PMID:
16314649.


14. Oh MG, Han MA, Park J, Ryu SY, Park CY, Choi SW. Health behaviors of cancer survivors: the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV, 2007-09). Jpn J Clin Oncol 2013;43:981-987. PMID:
23975890.



15. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-753. PMID:
18212285.


17. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49-S73. PMID:
24222018.


18. Eakin EG, Youlden DR, Baade PD, Lawler SP, Reeves MM, Heyworth JS, et al. Health behaviors of cancer survivors: data from an Australian population-based survey. Cancer Causes Control 2007;18:881-894. PMID:
17638108.



19. Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med 2005;40:702-711. PMID:
15850868.


20. Grimmett C, Wardle J, Steptoe A. Health behaviours in older cancer survivors in the English Longitudinal Study of Ageing. Eur J Cancer 2009;45:2180-2186. PMID:
19328679.


21. Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000;88:674-684. PMID:
10649263.


22. Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 2004;22:439-445. PMID:
14691120.


23. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB 3rd, Macdonald JS, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003;98:484-495. PMID:
12879464.


24. Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev 2005;14:2009-2014. PMID:
16103453.


25. Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 2005;23:4742-4754. PMID:
16034050.


26. Trivers KF, De Roos AJ, Gammon MD, Vaughan TL, Risch HA, Olshan AF, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol 2005;3:225-230. PMID:
15765441.


27. Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol 2000;18:1725-1732. PMID:
10764433.


28. Liu J, Hong Y, D'Agostino RB Sr, Wu Z, Wang W, Sun J, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA 2004;291:2591-2599. PMID:
15173150.


29. Ahn KA, Yun JE, Cho ER, Nam CM, Jang Y, Jee SH. Framingham equation model overestimates risk of ischemic heart disease in Korean men and women. Korean J Epidemiol 2006;28:162-170.