|
 |
- Search
| Korean J Fam Med > Volume 39(2); 2018 > Article |
Abstract
Background
Although chronic obstructive pulmonary disease is a known cause of secondary polycythemia with elevated erythropoietic demands in response to hypoxemia, anemia is relatively common in patients with chronic obstructive pulmonary disease and is related to increased mortality. However, little is currently known about the relationship between various iron parameters and disease severity in chronic obstructive pulmonary disease patients.
Methods
Data from the fifth Korean National Health and Nutrition Examination Survey, a population-based epidemiologic survey conducted in 2010ŌĆō2012, were used. A total of 1,129 patients with chronic obstructive pulmonary disease were examined to reveal the associations between the forced expiratory volume in 1 second (FEV1) and hemoglobin and iron parameters, including serum iron, ferritin, total iron binding capacity, and transferrin saturation, using Spearman correlations and multiple linear regression analyses.
Results
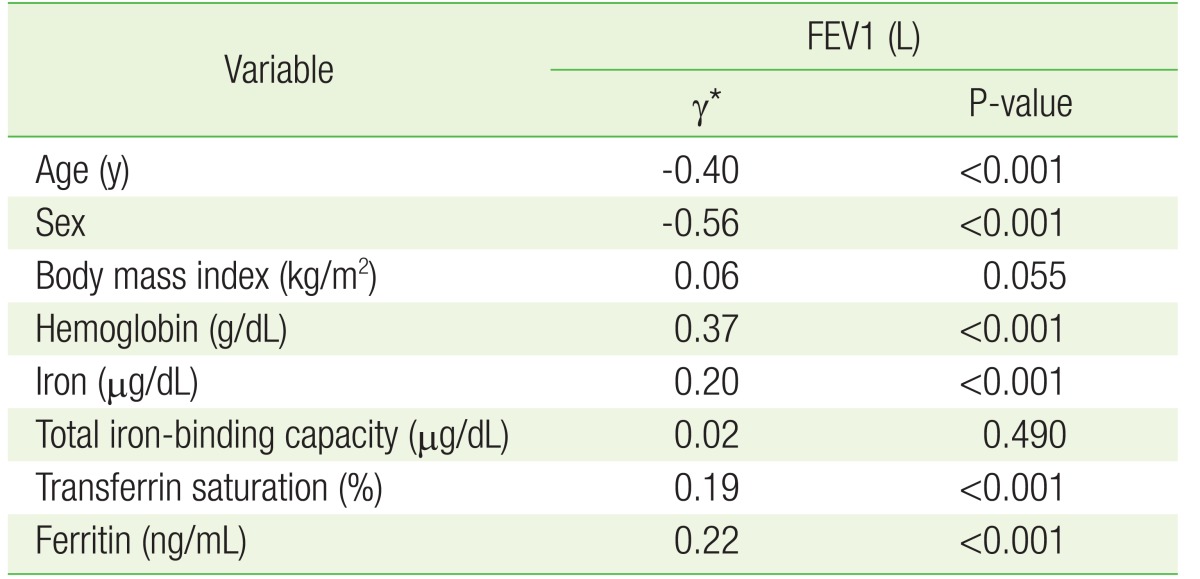
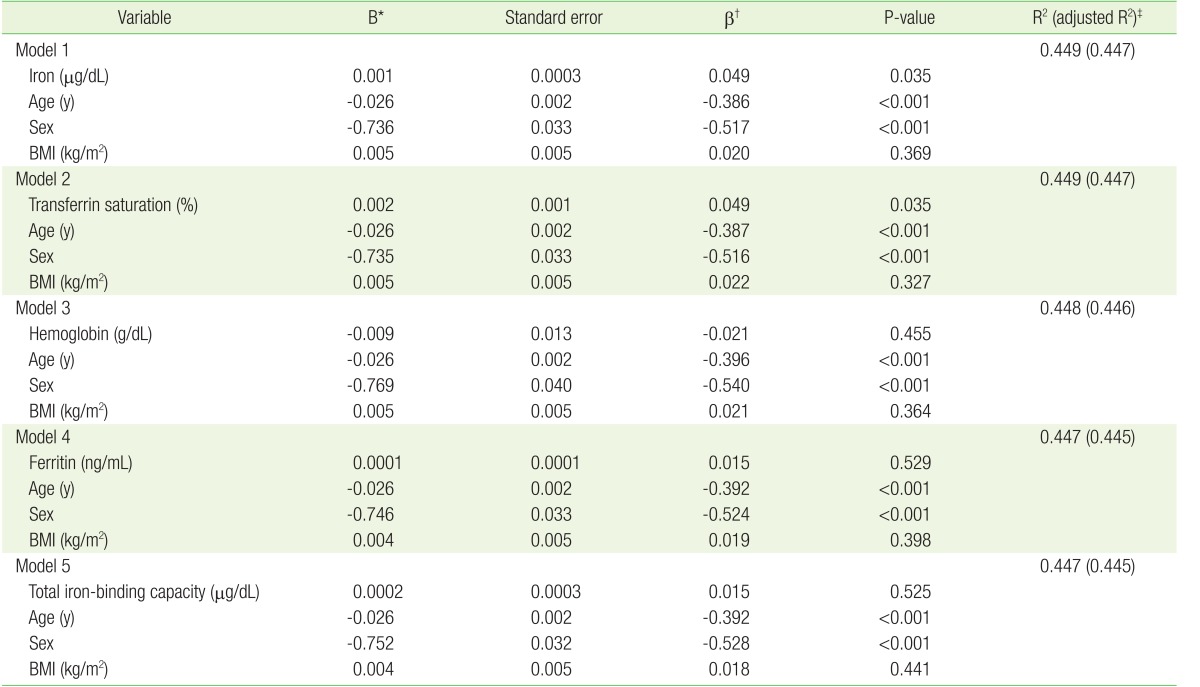
The FEV1 was positively correlated with serum hemoglobin (╬│=0.37, P<0.001), iron (╬│=0.20, P<0.001), transferrin saturation (╬│=0.19, P<0.001), and ferritin (╬│=0.22, P<0.001), and negatively correlated with age (╬│=ŌłÆ0.40, P<0.001) and lower in female patients (╬│=ŌłÆ0.56, P<0.001) in the Spearman correlation. The FEV1 was independently associated with serum iron (╬▓=0.049, P=0.035) and transferrin saturation (╬▓=0.049, P=0.035) after adjusting for age, sex, and body mass index in the multiple linear regression analyses.
Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation of the airways, lung parenchyma, and pulmonary vasculature, causing airflow limitation that is not fully reversible.1) COPD is a known cause of secondary polycythemia with elevated erythropoietic demands in response to the consequent hypoxemia. However, anemia is relatively common in patients with COPD and is related to increased mortality.2) Indeed, anemia-accompanied COPD has been shown to be independently related to incremental costs of $3,582 per patient per year according to data from United States Medicare claims.3)
A number of previous studies have shown decreased iron availability in COPD patients. Low-grade systemic inflammation in COPD may represent a possible cause of anemia in this chronic disease.4) More specifically, impaired iron availability results from upregulation of ferritin mRNA due to elevations in the levels of inflammatory cytokines such as interleukin-1 and interferon-c.5) In addition, certain inflammatory cytokines, such as tumor necrosis factor and interleukin-1, are thought to be related to reduced iron utilization,6) and one study suggested the presence of erythropoietin resistance in anemic COPD patients presenting with high erythropoietin levels, a phenomenon that is probably regulated through inflammatory mechanisms.7)
The iron status in patients with COPD reflects the balance between the stimulation of erythropoiesis by hypoxia and its depression by inflammation. The present study was conducted to determine whether the various iron parameters are associated with the severity of COPD. We measured the serum hemoglobin (Hb) and several iron parameters, including serum iron, ferritin, transferrin saturation (TSAT), and the total iron-binding capacity (TIBC), in patients with COPD, and their relation to the forced expiratory volume in 1 second (FEV1), as an indicator of COPD severity.
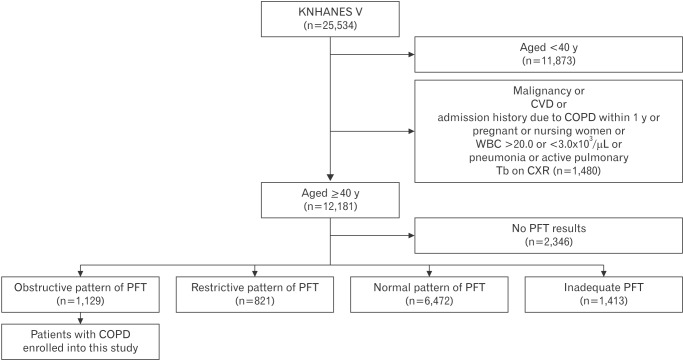
We performed a cross-sectional cohort study using data from the fifth Korean National Health and Nutrition Examination Survey (KNHANESV), a nationally representative stratified random sample of the Korean population in 2010ŌĆō2012. A flowchart for the study cohort selection procedure is presented in Figure 1. The survey involved subjects aged Ōēź40 years who had undergone complete spirometry measurements. The exclusion criteria included malignancy, cardiovascular disease, admission history due to COPD within the past year, pregnant or nursing women, white blood cell count of Ōēź20.0 or Ōēż3.0├Ś103┬Ą/mL, pneumonia or active pulmonary tuberculosis on chest radiograph, a restrictive or normal pattern identified by pulmonary function test (PFT), or an inadequate PFT. The clinical diagnosis of COPD was confirmed using the Global Initiative for Chronic Obstructive Lung Disease criteria.8) COPD was defined as an FEV1/forced vital capacity ratio <0.70.
We examined the Hb level and iron status using laboratory measures for serum iron, ferritin, and TIBC. TSAT was calculated as follows: TSAT (%)=serum iron├ĘTIBC├Ś100.
Data analyses were performed using IBM SPSS Statistics ver. 23.0.0.0 (IBM Corp., Armonk, NY, USA). The associations between FEV1 and the variables were evaluated using Spearman correlations. Multiple linear regression analysis was used to evaluate the relationships between FEV1 and the variables after adjusting for age, sex, and body mass index (BMI). Normally distributed continuous variables are expressed as the mean┬▒standard deviation. Non-normally distributed continuous variables are expressed as the median (minimumŌĆōmaximum) value. For all analyses, a P-value of less than 0.05 was considered statistically significant.
The baseline characteristics of the cohort are shown in Table 1. The median age of the cohort was 66 years (range, 40ŌĆō91 years). There were fewer female (27.55%) than male (72.45%) patients with COPD. The proportion of smokers (68.11%) was higher than that of never-smokers (30.29%). The median (minimumŌĆōmaximum) number of smoking pack-years was 15 (0ŌĆō180).
Table 2 shows the Spearman correlation analyses between FEV1 and the other variables, including Hb and iron parameters, in patients with COPD. The FEV1 was positively correlated with serum Hb (╬│=0.37, P<0.001), iron (╬│=0.20, P<0.001), TSAT (╬│=0.19, P<0.001), and ferritin (╬│=0.22, P<0.001), and negatively correlated with age (╬│=ŌłÆ0.40, P<0.001) and lower in female patients (╬│=ŌłÆ0.56, P<0.001).
Table 3 shows the multiple linear regression analyses between FEV1 and the variables, including serum Hb and iron parameters (iron, TIBC, TSAT, and ferritin), after adjustment for age, sex, and BMI. The multiple linear regression revealed serum iron (╬▓=0.049, P=0.035) and TSAT (╬▓=0.049, P=0.035) were independent determinants of FEV1 as an indicator of lung function after adjustment for age, sex, and BMI (models 1 and 2). Although the levels of Hb, ferritin, and TIBC significantly correlated with FEV1 in the Spearman correlation analyses (Table 2), they did not correlate with FEV1 after adjustment for age, sex, and BMI in the multiple linear regression analyses (Table 3: models 3, 4, and 5).
In this nationwide study, the serum iron and TSAT levels were found to be positively related with the FEV1 as an indicator of COPD severity after adjustment for age, sex, and BMI; that is, serum iron and TSAT negatively correlated with the severity of COPD. This finding indicates that the serum iron and TSAT levels could represent biomarkers of COPD severity.
In this study, absolute FEV1 was used to represent the severity of COPD and lung function instead of predicted FEV1. The severity of airflow limitation in COPD is stratified by the percentage of the predicted FEV1 in the GOLD classification.8) However, application of the predicted FEV1 levels may lead to inappropriate assessments of severity across different ethnicities, age groups, and sexes. Although patients may have the same absolute FEV1 levels, they may be categorized into different COPD severity levels depending on the reference equations.9) Miller and Pedersen10) found that using absolute FEV1 levels could better predict the survival rate compared with the predicted FEV1 values. Based on our results herein, the serum iron and transferrin saturation levels could be considered as biomarkers to support lung function as assessed by FEV1 in COPD patients.
This present study revealed that serum iron and TSAT were associated with FEV1 after adjustment for age, sex, and BMI in COPD patients using nationwide data. In a previous study, iron status was found to be associated with lung function and disease progression in COPD, with lower serum iron and TSAT associated with elevated mortality in patients with chronic respiratory failure. In addition, the authors also found that TSAT and Hb were independently associated with dyspnea.11) Another study showed that reduced iron intake was related with an increased risk of COPD and decreased percentage of the predicted FEV1, though the correlation coefficients were low.12)
In this study, low serum iron and TSAT were related with severe COPD. COPD represents a generalized inflammatory status with elevated production of acute-phase proteins, such as ferritin and hepcidin, and inhibited iron mobilization from reticuloendothelial iron stores, which could result in a functional iron deficiency (ID) despite high ferritin levels. Currently, a ferritin cut-off level of <12 ng/mL is considered to have a very high specificity for the prediction of absolute ID;13) however, the criterion for anemia should be different in patients with COPD compared with that in healthy individuals, because the pathogenesis of COPD involves a combination of inflammation, hypoxemia, and ID. Thus, because there are currently no definite clinical Hb or iron parameter cut-off levels for detecting anemia among COPD patients, further studies are needed.
Several studies have shown that the treatment of anemia or ID should be considered in COPD patients. Non-anemic ID was found to be related to decreased aerobic capacity and a lower pulmonary rehabilitation response in COPD patients compared with in patients with normal iron status in one previous study.14) Further, some studies have shown the benefits of iron supplementation in chronic inflammatory disease states such as COPD, congestive heart failure, and chronic kidney disease. Indeed, intravenous iron treatment has been shown to improve functional capacity and quality-of-life in chronic kidney disease15) and heart failure patients with ID, either with or without anemia.16,17) Furthermore, Silverberg et al.18) suggested that dyspnea in COPD patients may be improved with the use of erythropoiesis-stimulating agents and intravenous iron to correct for ID.
Serum iron and TSAT have several advantages as biomarkers in COPD, including cost-effectiveness, simplicity, their significant relationship with FEV1, and relationship with the treatment. Nevertheless, before routinely using iron supplementation as a treatment, further studies should be conducted to examine the risks and effects of iron overload toxicity and the appropriate amount of iron for the treatment of ID in COPD patients. Further, the fact that polycythemia can lead to pulmonary hypertension and venous thromboembolism should be considered.19)
There were some limitations to the present study. Because the KNHANES V did not collect data on the C-reactive protein levels, we were inevitably not able to exclude subjects with elevated C-reactive protein levels, which indicate an acute inflammatory state. To resolve this limitation, we excluded subjects with elevated white blood cell levels as a marker of the inflammation state. Another major limitation is the fact that reversibility tests using bronchodilators were not performed, hence resulting in potential overlap with asthma. However, chronic asthma may also demonstrate traits of irreversible airflow obstruction and thus be included in the definition of ŌĆ£COPD.ŌĆØ20)
In conclusion, the serum iron (╬▓=0.049, P=0.035) and TSAT (╬▓=0.049, P=0.035) levels were independently associated with FEV1 as a marker of COPD severity.
References
1. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256-1276. PMID: 11316667.


2. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis 2011;6:199-208. PMID: 21660297.


3. Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood 2011;118:4129-4139. PMID: 21873546.


4. Similowski T, Agusti A, MacNee W, Schonhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J 2006;27:390-396. PMID: 16452598.


5. Rogers J, Lacroix L, Durmowitz G, Kasschau K, Andriotakis J, Bridges KR. The role of cytokines in the regulation of ferritin expression. Adv Exp Med Biol 1994;356:127-132. PMID: 7534028.


6. Means RT Jr. Advances in the anemia of chronic disease. Int J Hematol 1999;70:7-12. PMID: 10446488.

7. Markoulaki D, Kostikas K, Papatheodorou G, Koutsokera A, Alchanatis M, Bakakos P, et al. Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Intern Med 2011;22:103-107. PMID: 21238904.


8. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-555. PMID: 17507545.


9. Checkley W, Foreman MG, Bhatt SP, Dransfield MT, Han M, Hanania NA, et al. Differences between absolute and predicted values of forced expiratory volumes to classify ventilatory impairment in chronic obstructive pulmonary disease. Respir Med 2016;111:30-38. PMID: 26712569.


10. Miller MR, Pedersen OF. New concepts for expressing forced expiratory volume in 1 s arising from survival analysis. Eur Respir J 2010;35:873-882. PMID: 19741033.


11. Schneckenpointner R, Jorres RA, Meidenbauer N, Kollert F, Pfeifer M, Budweiser S. The clinical significance of anaemia and disturbed iron homeostasis in chronic respiratory failure. Int J Clin Pract 2014;68:130-138. PMID: 24341307.


12. Hirayama F, Lee AH, Oura A, Mori M, Hiramatsu N, Taniguchi H. Dietary intake of six minerals in relation to the risk of chronic obstructive pulmonary disease. Asia Pac J Clin Nutr 2010;19:572-577. PMID: 21147720.

13. Cavill I. Iron status as measured by serum ferritin: the marker and its limitations. Am J Kidney Dis 1999;34(4 Suppl 2):S12-S17. PMID: 10516370.


14. Barberan-Garcia A, Rodriguez DA, Blanco I, Gea J, Torralba Y, Arbillaga-Etxarri A, et al. Non-anaemic iron deficiency impairs response to pulmonary rehabilitation in COPD. Respirology 2015;20:1089-1095. PMID: 26148453.


15. Locatelli F, Barany P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013;28:1346-1359. PMID: 23585588.


16. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436-2448. PMID: 19920054.


17. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008;51:103-112. PMID: 18191732.


18. Silverberg DS, Mor R, Weu MT, Schwartz D, Schwartz IF, Chernin G. Anemia and iron deficiency in COPD patients: prevalence and the effects of correction of the anemia with erythropoiesis stimulating agents and intravenous iron. BMC Pulm Med 2014;14:24PMID: 24564844.



19. Nand S, Orfei E. Pulmonary hypertension in polycythemia vera. Am J Hematol 1994;47:242-244. PMID: 7942794.


20. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;152(5 Pt 2):S77-S121. PMID: 7582322.

Figure┬Ā1
Flowchart for the study cohort selection procedure. KNHANES V, fifth Korean National Health and Nutrition Examination Survey; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; WBC, white blood cells; Tb, tuberculosis; CXR, chest X-ray; PFT, pulmonary function test.
Table┬Ā1
Baseline characteristics of patients with COPD (N=1,129)
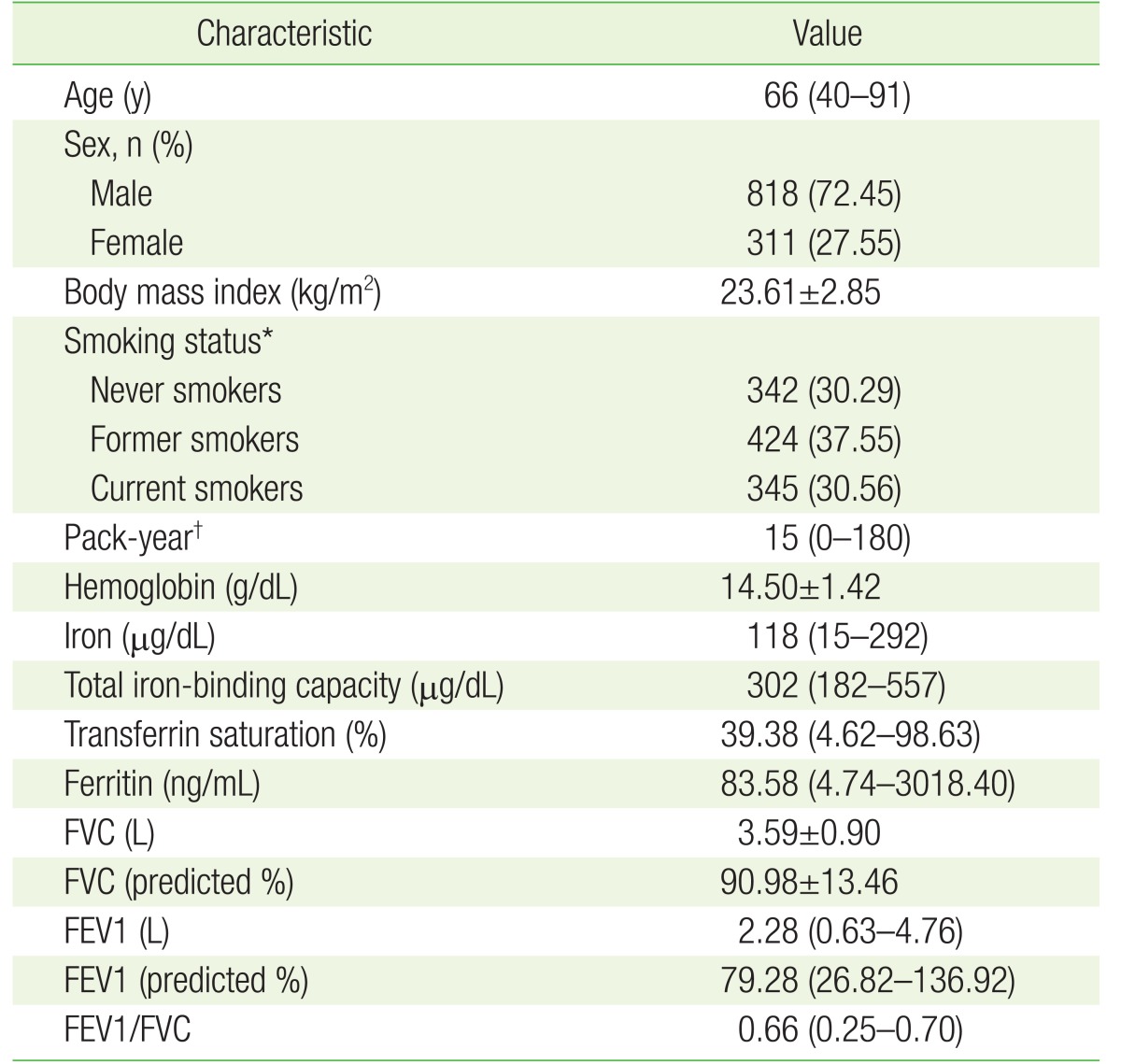
Values are presented as median (range), number (%), or mean┬▒standard deviation.
COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second.
*Data regarding smoking status were missing for 18 subjects. ŌĆĀData regarding packyears were missing for 19 subjects.
Table┬Ā2
Correlations between FEV1 and other variables in patients with chronic obstructive pulmonary disease