The Relationship between Serum Gamma-glutamyltransferase Level and Overweight in Korean Urban Children
Article information
Abstract
Background
Recently, it has been reported that gamma-glumyltransferase (GGT) is associated with various cardiovascular risk factors including overweight in adults. However, there are few studies on the relationship between GGT and cardiovascular risk factors in children. The aim of this study was to assess the relationship between serum GGT level and overweight in Korean urban children.
Methods
This study was a cross-sectional study using data on 390 4th grade students of elementary schools in Gunpo, Korea. Children were divided into 4 groups according to gender-specific quartiles of serum GGT level. Body mass index, waist circumference and body fat percentage were quantified as adiposity indices.
Results
All adiposity indices in children of the highest GGT level quartile were higher than those in children of the lowest quartile. Adjusted odd ratios on overweight of the highest quartile of GGT level compared to the lowest quartile were 14.40 (95% confidence interval [CI], 4.43 to 46.83) in boys and 2.94 (95% CI, 1.06 to 8.16) in girls.
Conclusion
This study shows that high serum GGT level is related with overweight in Korean urban children and this relationship is stronger in boys compared to girls.
INTRODUCTION
Childhood obesity is rapidly becoming worldwide from Western countries to Asian countries.1-3) Obesity tends to track from childhood to adulthood and childhood obesity can be a predictor of cardiovascular disease during adulthood.4) Additionally, epidemic of childhood obesity leads to early development of hypertension, type 2 diabetes, dyslipidemia and fatty liver in childhood.5,6) This increasing trend of obesity and obesity-related diseases during childhood have been a threat to the public health.
Gamma-glutamyltransferase (GGT) has traditionally been used as a marker of excessive drinking or hepatobiliary disease. However, the relationship between GGT and cardiovascular disease has been recently reported in adults. It was reported that GGT is related to cardiovascular risk factors such as obesity, smoking, sedentary lifestyle habits, dyslipidemia, hypertension, type 2 diabetes and metabolic syndrome in cross-sectional studies7,8) and can be a predictor of the incidence and prognosis of hypertension, type 2 diabetes, coronary heart disease and stroke in prospective studies.9-11) Additionally, a great deal of evidence has been presented that GGT is related to oxidative stress.12-14) Most studies on GGT have been conducted on adults. Children are suitable for the study on the relationship between GGT and cardiovascular disease risk factors such as overweight because children have rarely been exposed to drinking, smoking, and other substances that can affect serum GGT level.
This study was conducted to investigate the relationship between serum GGT level and overweight in Korean children. The cross-sectional data from 390 4th grade students of elementary schools in Gunpo, Korea were used for analyses.
METHODS
In 2006, 538 children were recruited from 4th grade students from 3 elementary schools in Gunpo, Korea. Among them, 134 children, who did not take blood tests, and 14 children, who had higher level of AST, ALT, and/or GGT than normal range (ALT, 5 to 45 U/L; AST, 15 to 55 U/L [nine-year-olds]; 5 to 45 U/L [ten-year-olds]; GGT, 5 to 32 U/L, presented by the Korean Pediatric Society)15) were excluded. Finally, the data of 390 children were available for the final analyses. Informed consent was obtained from their parents.
Information on parental height and weight, total household income and birth weight was obtained by self-administered questionnaires. Questionnaires were asked to fill out by children and their parents together. Parental body mass index (BMI) was calculated with self-reported height and weight.
Anthropometric data were collected in the morning after overnight fasting, by well-trained nurses under surveillance of an investigator in charge. All anthropometric measurements were done on bare feet and with light clothing. Height was measured by manual height scale. Body weight and body fat percentage (BF%) were measured by a bioelectrical body composition analyzer (Genius-220, Jawon Medical, Kyungsan, Korea). Waist circumference was measured at the midpoint between lower margin of 12th rib and iliac crest after normal expiration with a non-elastic tape. BMI was calculated by following formula: body weight (kg)/height (m)2. We define overweight as BMI ≥ 85th percentile for each age and gender based on the 1998 growth chart for Korean children aged 2-18 years.16) Blood pressure was measured in stable condition by a mercury sphygmomanometer with cuff for children.
Blood tests were taken only in children with parental consent. Fasting blood samples were done on antecubital vein with a disposable syringe. GGT, AST, ALT, fasting glucose, total cholesterol (T-Chol), high density lipoprotein cholesterol (HDL-C) and triglyceride (TG) were measured with an auto-analyzer (Selectra 2, Merck, Dieren, The Netherland).
GGT, AST, ALT and TG were analyzed after log transformation because they did not have a normal distribution. Study subjects were divided into 4 groups according to gender-specific quartiles of serum GGT level (Boys Group 1: ≤12.2 mg/dL, Group 2: 12.3 to 13.8 mg/dL, Group 3: 13.9 to 15.7 mg/dL, Group 4: ≥15.8 mg/dL; Girls Group 1: ≤ 12.0 mg/dL, Group 2: 12.1 to 13.0 mg/dL, Group 3: 13.1 to 14.6 mg/dL, Group 4: ≥ 14.7 mg/dL). Adiposity indices and cardiovascular risk factors according to gender specific quartiles of serum GGT level were compared by one-way ANOVA and post-hoc Tukey test. The odds ratio (OR) on overweight was calculated in each group according to serum GGT level using the multiple logistic regression analysis with adjustments for age, birth weight, maternal weight status and total household income. All statistical analyses were performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) and values with P < 0.05 (two-tailed tests) were considered significant.
RESULTS
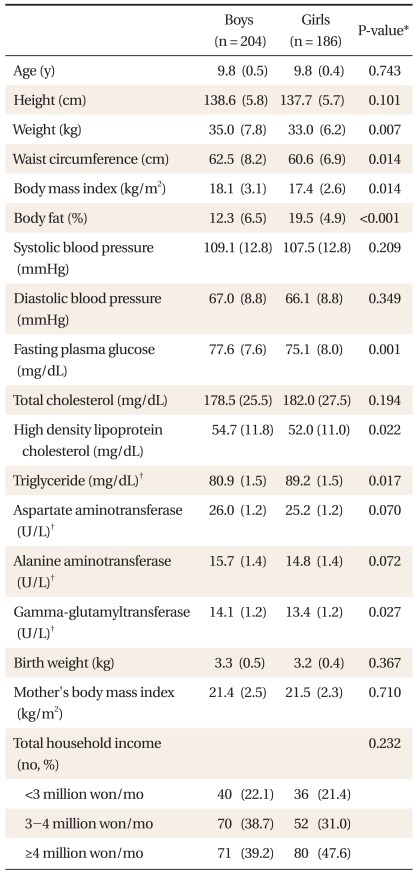
Out of 390 children (boys, 204; girls, 186) participating in this study, the prevalence rate of overweight was 26.7% (boys, 27.9%; girls, 25.2%) and geometric mean level of GGT was 13.7 ± 1.2 U/L (boys, 14.1 ± 1.2 U/L; girls, 13.4 ± 1.2 U/L). General characteristics of study subjects were presented in Table 1.
According to gender-specific quartile of serum GGT level, adiposity indices such as BMI, waist circumference and BF% and cardiovascular risk factors, such as blood pressure, fasting glucose, T-Chol, HDL-C, and TG, were compared. All adiposity indices were higher in children in the group with the highest GGT level than children in the group with the lowest in both genders. The differences of adiposity indices between children of those 2 groups were more remarkable in boys than in girls (Figure 1). Boys in the group with the highest GGT level had a higher mean level of systolic blood pressure, diastolic blood pressure, TG and ALT; girls in the group with the highest GGT level had a higher mean level of T-Chol and ALT compared to those with the lowest GGT level (Table 2).
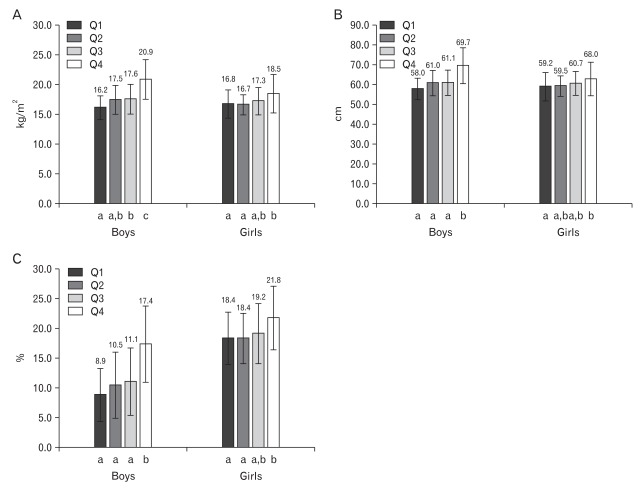
Comparison of adiposity indices according to gender-specific quartiles of serum gamma-glutamyltransferase (GGT) level. (A) Body mass index (kg/m2). (B) Waist circumference (cm). (C) Body fat (%). All P < 0.05 by one-way ANOVA. Error bars represent ± SD. Values marked with a different letter are significantly different (P < 0.05) by post-hoc Tukey test. Boys Q1: ≤12.2 mg/dL, Q2: 12.3-13.8 mg/dL, Q3: 13.9-15.7 mg/dL, Q4: ≥15.8 mg/dL; Girls Q1: ≤12.0 mg/dL, Q2: 12.1-13.0 mg/dL, Q3: 13.1-14.6 mg/dL, Q4: ≥14.7 mg/dL.
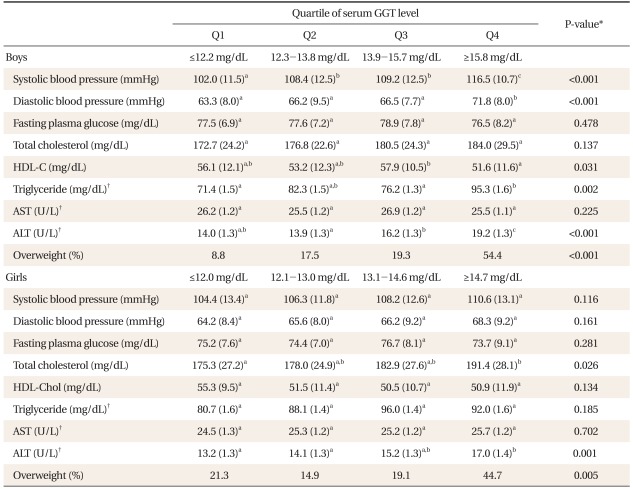
Comparison of cardiovascular risk factors according to gender-specific quartiles of serum Gamma-glutamyltransferase (GGT) level.
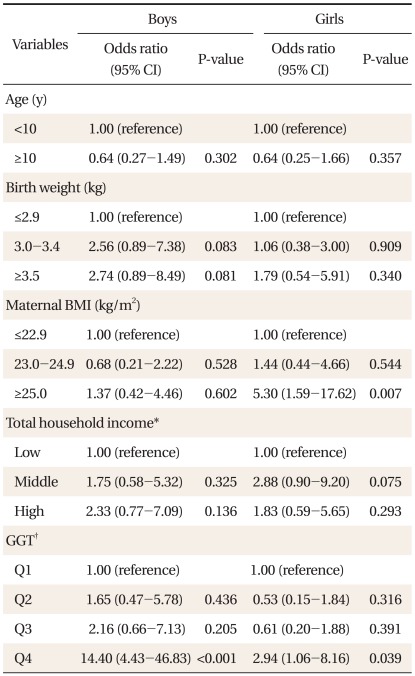
Multiple logistic regression analysis was performed to elucidate which serum GGT level was independently related to overweight in children. Prevalence of overweight was higher in children of group with the highest GGT level than children of group with the lowest in both genders. Using the lowest quartile of GGT level as reference, adjusted ORs of the highest quartile were 14.40 (95% confidence [CI], 4.43 to 46.83) in boys and 2.94 (95% CI, 1.06 to 8.16) in girls (Table 3).
DISCUSSION
We investigated the relationship between serum GGT level and overweight in Korean urban children. A series of evidences have been presented recently that serum GGT level is associated with various cardiovascular risk factors including overweight and metabolic syndrome and can predict the incidence and prognosis of type 2 diabetes and cardiovascular diseases.17) However, most of these studies have been conducted on adults. Li et al.18) reported that ALT and GGT were related to obesity and triglyceride in aboriginal children in Taiwan; Ortega et al.19) also reported that serum GGT level among liver enzymes was the only independent factor related to insulin resistance in Pima Indian children. Results of these previous studies were consistent with that of the present study.
Liver steatosis was considered as a mechanism for the relationship between serum GGT level and cardiovascular risk factors including overweight. Fatty liver disease in children has been increasing along with the increase of childhood obesity.5,20) However, several studies in adults presented that this relationship can not be explained by liver steatosis alone.10) In our study, the relationship between GGT and overweight was observed in children with a normal level of ALT, which increase before GGT in the development of fatty liver. Even after ALT level was adjusted in the multiple logistic regression analysis, the relationship between serum GGT level and overweight did not change (data not shown). These results indicate that the relationship between GGT and overweight cannot be explained solely by fatty liver.
Study results on the association between GGT and oxidative stress have been presented. Lee et al.14,21) showed that the association between low levels of serum anti-oxidative enzymes and high level of serum GGT, as well as with that high intake of foods containing antioxidants and low level of serum GGT. Oxidative stress induce the increase of GGT, which causes reuptake of glutathione into cells.12) However, Cysteinyl-glycine, which is produced in this process, generates reactive oxygen species (ROS) through reaction with iron out of cells. Thereafter, generated ROS catalyzes oxidation of low density lipoprotein cholesterol (LDL-C) and causes progression of atherosclerosis.13)
It was already known that oxidative stress has a role in the development of metabolic diseases such as insulin resistance, type 2 diabetes, hypertension, dyslipidemia, and atherosclerosis.22,23) An experimental study showed that obesity generates ROS by reducing NADPH oxidase and several anti-oxidative enzyme hormones.24) It was observed in an epidemiologic study that obesity contributes to the development of cardiovascular diseases by increasing oxidative stress.25) Study results on the association between oxidative stress and obesity and metabolic diseases have recently been released. Atabek et al.26) reported that obese children had higher D-Roms (an index of oxidative stress) than normal weight children, and D-Roms had a significant relationship with blood pressure and cholesterol. Mohn et al.27) reported that malondialehyde (MDA), an index of lipid peroxdiation, was higher in obese children than in normal weight children and their MDA level was decreased to those of normal weight children through a dietary restriction-weight loss program for six months.
Erdeve et al.28) reported that obese children had a higher level of superoxide dismutase, an anti-oxidant enzyme, compared to normal weight children; Mandato et al.29) reported that children with fatty liver disease had an elevated level of glutathione peroxidase. These study results in children seems to be against those in adults, in which obese men had a decreased level of anti-oxidant enzymes. This difference between studies results in children and adults could be interpreted as the following; anti-oxidant enzymes increase to defend against increased oxidative stress in obese children, whereas in obese adults, anti-oxidant enzymes could decrease because their protective mechanism against oxidative stress breaks down by exposure to oxidative stress for a long time.
This study has some limitations to be considered. There was insufficient research on the past medical history such as viral hepatitis and medication that can affect serum GGT level. We did not include children with abnormal level of AST, ALT, and GGT in order to exclude the cases of liver injuries caused by viral hepatitis or toxic hepatitis. The results of this study cannot be generalized because this study included only 4th grade students of elementary schools in one city. Cross-sectional design of this study prevented causal inferences.
This study showed that serum GGT level was related to overweight and various cardiovascular risk factors in Korean urban children. The relationship between serum GGT level and overweight was greater in boys than in girls. A series of studies on the steatohepatitis reported that the prevalence of fatty liver was higher in boys than girls.30) The vulnerability to the development of fatty liver in boys could be a possible cause of greater relationship between serum GGT level and overweight in boys. Additionally, boys could have a more sensitive reaction to an increased oxidative stress caused by overweight compared to girls.
Although GGT is an indicator of liver steatosis and increased oxidative stress, chronic increase of GGT itself can lead to oxidative stress and increase the risk of cardiovascular diseases. Therefore, it is thought that overweight children with continuously elevated level of serum GGT may need intervention for weight reduction in order to reduce the risk of cardiovascular diseases.