The Association between Symptoms of Autonomic Neuropathy and the Heart Rate Variability in Diabetics
Article information
Abstract
Background
There are few tools to detect the diabetic autonomic neuropathy at an earlier stage. This study was conducted to investigate the association between symptoms of autonomic neuropathy and the heart rate variability (HRV) in diabetics.
Methods
Study subjects consisted of 50 diabetic patients and 30 outpatient hospital control patients at a university family medicine department. The patients completed a Korean version of composite autonomic symptom scale (COMPASS). Electrocardiography was recorded in the supine position, on standing, and during deep breathing, for 5 minutes each. HRV of frequency domain was calculated by power spectral analysis.
Results
The COMPASS score was higher in female diabetic patients compared with that in controls. Among 50 diabetic patients, the total COMPASS score correlated positively with normalized low frequency (LF) score (normalized units, n.u.) (r = 0.62, P < 0 .001) and low frequency/high frequency (LF/HF) (r = 0.77, P < 0.001), negatively with normalized HF score (n.u.) (r = -0.59, P < 0.001) and RMSSD (square root of the mean of the sum of the square of differences between adjacent NN interval; r = -0.33, P = 0.031). The decrease in LF (n.u) and the increase in HF (n.u) by deep breathing from the supine position were higher in diabetic patients compared with those in controls. The increase in LF (n.u) and the decrease in HF (n.u) by standing from the supine position were lower in diabetic patients compared with those in controls.
Conclusion
The COMPASS score correlated with some component score of the HRV in diabetics. The HRV may be used as a tool to detect diabetic autonomic neuropathy by augmentation with position change.
INTRODUCTION
Diabetic autonomic neuropathy may occur at any stage of diabetes, but it usually develops in patients who have had diabetes for 20 years or more.1) Cardiovascular autonomic neuropathy may result in orthostatic hypotension, persistent sinus tachycardia, and asymptomatic myocardial infarction2) which may predispose sudden death.3-5)
Therefore careful history taking and a thorough physical examination are important to screen the presence of diabetic neuropathy at an early stage of diabetes. Diagnosis of diabetic autonomic neuropathy primarily depends on several autonomic symptoms, but few studies validated symptom profile as a diagnostic tool.6,7)
The heart rate variability (HRV) is a physiological phenomenon which reflects the variety of time intervals between heart beats. It is measured by the variation in the beat-to-beat interval. The main inputs to make a HRV are the balance between the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS). By detecting HRV, we can compare the relative activity of the SNS with the PNS. The HRV is also used to evaluate the cardiovascular autonomic regulation.8)
Power spectral analysis demonstrates the HRV as a time domain and a frequency domain. As a time domain, RMSSD (square root of the mean of the sum of the square of differences between adjacent NN interval) reflects the parasympathetic activity. As a frequency domain, high frequency (HF) activity (0.4 to 0.15 Hz), especially, has been linked to PNS activity. Less is known about the physiological inputs of the low frequency (LF) activity (0.04 to 0.15 Hz), though recent consensus suggests it is influenced either by the SNS or a mixture of both the SNS and the PNS. The LF/HF ratio is known to be influenced by the sympathovagal balance.9,10)
The composite autonomic symptom scale (COMPASS) is a self-completed questionnaire that is composed of 73 items. These questions are important and critical in the diagnosis of autonomic neuropathy.11) The COMPASS has nine weighted subscale scores and total score is calculated by summing the individual subscale score. It has been proved to correlate objective indexes of autonomic function and autonomic symptom severity.12) The HRV as a tool to measure autonomic balance may be related with the COMPASS symptom profile, but there was no study about it. If we know the association between these two measurements, we can use them complementary to each other because one is symptom-based, and the other is autonomic sign-based.
This study was conducted to investigate the association between symptoms of autonomic neuropathy and the HRV in diabetics.
METHODS
1. Subjects
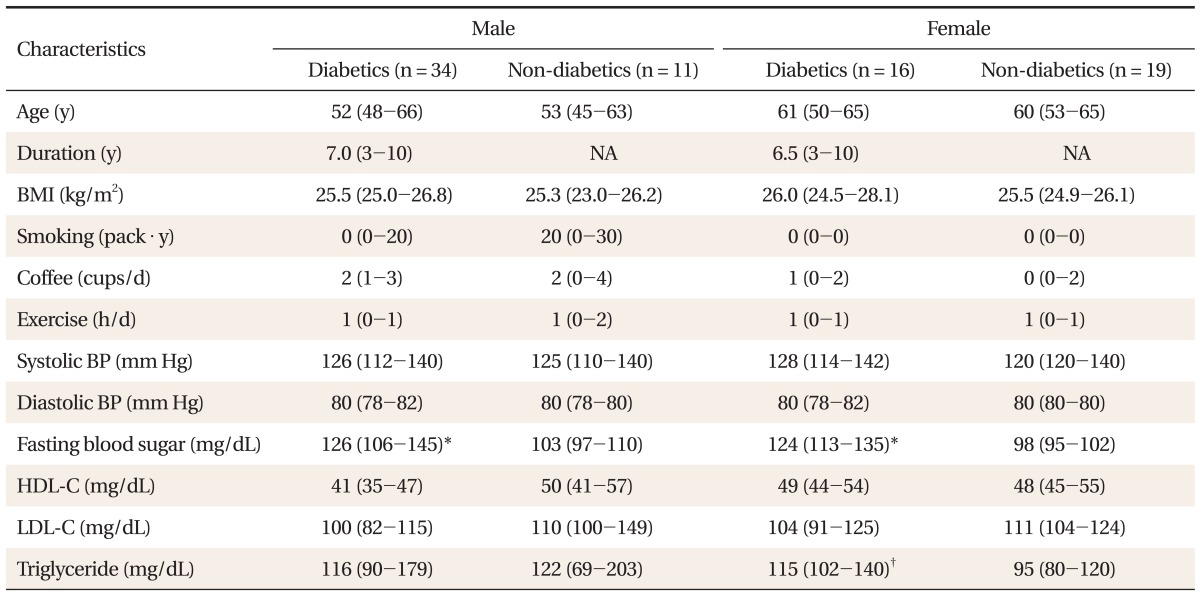
Study subjects consisted of 50 diabetic patients (34 male, 16 female) and 30 outpatient hospital control patients (11 male, 19 female) at a university family medicine department from March 2008 through August 2008. Age of the study subjects ranged from 42 to 74 years old. Diagnosis and classification of diabetes were based on guidelines of the Expert Committee Report of the American Diabetes Association.13)
We excluded those with known arrhythmia, unstable angina, myocardial infarction, or cerebrovascular disease, and those who were taking any autonomic drug such as β-blocker, β-agonist, calcium channel blocker, or angiotensin-converting enzyme inhibitors, etc. The written consent was obtained from each patient before enrollment in the study. This study was approved by the Institutional Review Board of Hanyang University Hospital, in Seoul, Korea. As baseline clinical characteristics, patient's age, sex, body mass index, smoking status, alcohol intake, exercise, and average consumption of coffee were assessed. Systolic and diastolic blood pressures were measured by a mercury sphygmomanometer. The serum levels of fasting blood glucose, total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol were measured by standard methods.
2. Measurement of Autonomic Symptoms and the HRV
1) COMPASS
The COMPASS has weighted 9 subscale scores that assess severity of symptoms in the following domains: orthostatic intolerance (9 items; range, 0 to 40), secretomotor dysfunction (8 items; range, 0 to 20), male sexual dysfunction(8 items; range, 0 to 30), bladder dysfunction (3 items; range, 0 to 20), gastrointestinal symptoms (14 items; range, 0 to 40), pupillomotor symptoms (7 items; range, 0 to 5), vasomotor symptoms (11 items; range, 0 to 10), reflex syncope (5 items; range, 0 to 20), and sleep dysfunction (8 items; range, 0 to 15). The range of item score is from the minimum score of two to the maximum score of seven in each item. The total scores are calculated by summation of the individual item scores and the maximum score may be up to 200 (in man) or 170 (in woman). All subjects completed the questionnaire within 5 minutes.
2) HRV
Study subjects got the blood test at fasting state for 8 hours, avoiding alcoholic drink, caffeine beverage, and excessive exercise for 24 hours before the test. The measurement was done from 9:00 AM to noon at room temperature of 22-24℃ in a quiet and comfortable environment. Participants took a rest for three minutes or more in the supine position and were oriented on the method of the test. Electrocardiography was recorded in the supine position, on standing, and during deep breathing (inspiration for 3 seconds and expiration for 3 seconds), for 5 minute each. The HRV of frequency domain was calculated by power spectral analysis.
3) HRV data analysis
The MATLAB ver. 7.04 (Mathwork Inc., Natick, MA, USA) reorganized the electrocardiography of each participant, then we deleted the abnormal heart beat and any artifact by using hand operated manual. Data of electrocardiography were digitalized by Analog to Digital Converter (MP150, BIOPAC Systems Inc., Goleta, CA, USA) and analyzed by the program of HRV Analysis ver. 1.1 (Department of Applied Physics, University of Kuopio, Eastern Finland, Finland). We calculated mean RR, RMSSD as a time domain, LF (0.04-0.15 Hz), HF (0.15-0.4 Hz), LF/HF ratio as a frequency domain by power spectral analysis. The LF and HF power were transformed into normalized units (n.u.). Transforming data into normalized units also helped to accentuate sympathovagal balance. Normalized units were calculated as follows: LF (n.u.) = LF power / TP-VLF power × 100; HF (n.u.) = HF power / TP-VLF power × 100 (TP, total power; VLF, very low frequency).
3. Statistical Analysis
All clinical data were summarized as median and interquartile range. Continuous clinical data were compared between diabetic patients and controls by using Mann-Whitney U test, Student's t-test. Partial correlation coefficient, Pearson r was calculated to examine the correlation between the total COMPASS score and HRV domain scores after adjusting confounding variables. We used SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). We considered it statistically significant if P-values were below 0.05.
RESULTS
1. General Characteristics of the Study Subjects
Diabetic patients had higher fasting glucose (P < 0.001) both in men and in women group, low serum triglyceride (P < 0.05) in women when compared with those of controls. All other clinical characteristics were not significantly different between diabetics and control group (Table 1).
2. The COMPASS Score
As a severity index of autonomic dysfunction, the total COMPASS score was higher in diabetic patients compared with that in controls. However, only female patients had significantly higher total COMPASS scores compared with the male controls (diabetics, 9.0; controls, 4.5; P < 0.05) (Table 2). Although data are not shown, among nine subscales, the subscale score of sleep dysfunction was significantly higher only in female diabetics compared with that of female control group (1.5 [range, 1 to 3] vs. 0 [range, 0 to 2]; P < 0.05).
3. HRV Domain Scores
In order to examine the characteristics of HRV domains (LF [ms2], LF [n.u.], HF [ms2], HF [n.u.], LF/HF, RMSSD [ms]) of diabetic patients, HRV domain scores in diabetic patients were compared with those of controls. All HRV domain scores were not significantly different between these two groups in the supine position, on standing, and during deep breathing (Table 2).
4. The Correlation between COMPASS Scores and Each of HRV Domain Scores
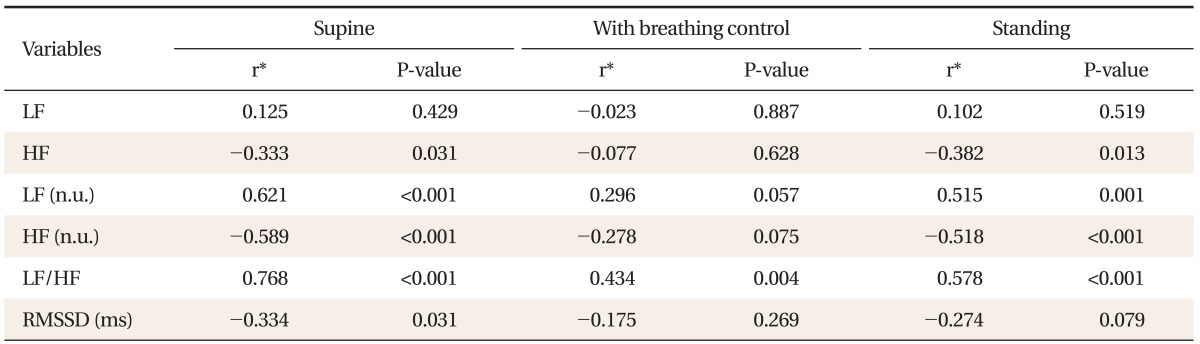
Partial correlation coefficient between total COMPASS score and HRV domain score was calculated after adjusting for age, sex, fasting blood glucose, body mass index, systolic blood pressure, diastolic blood pressure, smoking, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglyceride. Among 50 diabetic patients, the total COMPASS score correlated positively with normalized LF score (n.u.) (r = 0.62, P < 0.001) and LF/HF (r = 0.77, P < 0.001), negatively with normalized HF score (n.u.) (r = -0.59, P < 0.001) and RMSSD (r = -0.33, P = 0.031) (Table 3).
5. Difference between HRV Domain Scores in Supine Position, in Supine Position with Deep Breathing, and on Standing
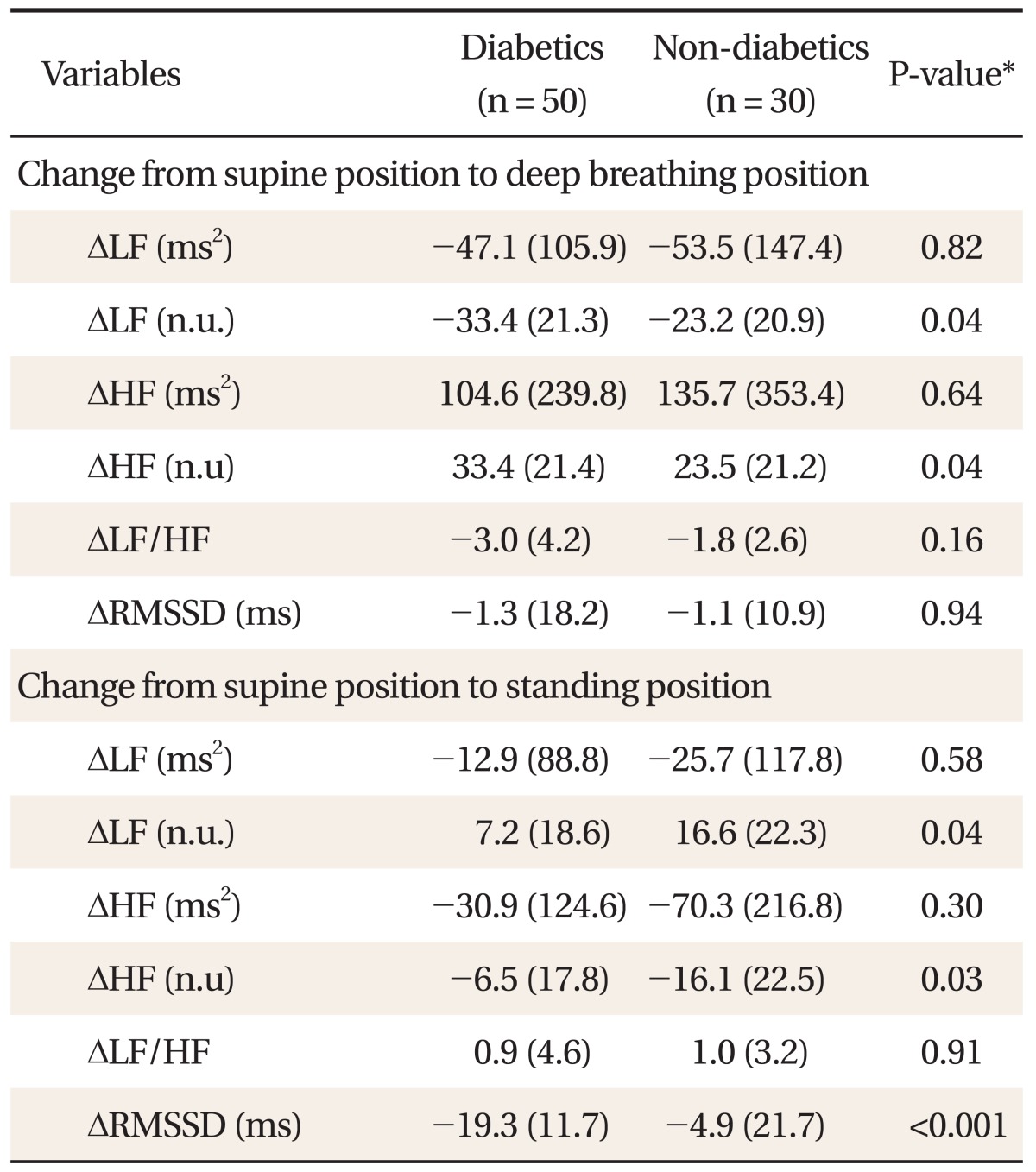
In order to compare HRV responses to deep breathing and standing in two groups, we measured the difference between HRV domain scores in the supine position, in the supine position with deep breathing, and on standing (Table 4). The decrease in LF (n.u) (P = 0.04) and the increase in HF (n.u) (P = 0.04) by deep breathing from the supine position were higher in diabetic patients compared with those in controls. The increase in LF (n.u) (P = 0.04) and the decrease in HF (n.u) (P = 0.03) by standing from the supine position were lower in diabetic patients compared with those in controls.
DISCUSSION
Diabetic autonomic symptoms and signs manifest themselves definitely in pupil, sweat gland, urinary system, gastrointestinal system, adrenal system, and cardiovascular system. Antagonism and balance between sympathetic nerve and parasympathetic nerve effect on these systems.14) The COMPASS is designed to provide a severity index of autonomic symptoms and gives different weights according to clinical importance. It yields one total score reflecting overall severity of autonomic symptoms.15) In this study, we found that autonomic symptoms among the diabetic patients were significantly prevalent compared with control subjects. Among nine subscales, the orthostatic intolerance was the most frequent symptom. As the item score of orthostatic intolerance was highly weighted to make a total COMPASS score, it may be regarded as one of important determining factors for the total COMPASS score.
The COMPASS score correlated with some component scores of the HRV in diabetics. HRV domains related to SNS (normalized LF score and LF/HF ratio) correlated positively, but HRV domains related to PNS (normalized HF score, HF score, and RMSSD) did negatively with the total COMPASS score. Therefore, it suggests that the higher prevalence of autonomic symptoms in diabetic patients is, the higher activity of SNS in these patients is expected. Degeneration of the vagus nerve in an early stage9,16) might result in relatively increased sympathetic activity at rest. It had been reported that high activity of SNS was the independent prognostic factor of hypertensive ventricular hypertrophy, morbidity and mortality of cardiovascular diseases.17-19) Enabling risk stratification by screening for cardiac autonomic diabetic neuropathy (CADN) might facilitate early and successful intervention, even in relation to CADN.20)
All of the HRV domain scores were not significantly different between the diabetic group and the control group. However, we found significant change of HRV response to deep breathing and standing in both groups. Generally, long expiration by deep breathing stimulates the PNS and results in increased HF score and decreased LF score.9,21) On the other hand, standing stimulates SNS and results in decreased HF score and increased LF score. Especially, we found the change of HRV scores was lower in diabetic patients by augmentation with positional change. Therefore, it may be interpreted that the diabetic patients had lowered sympathetic responses to positional change like standing20,22) and increased orthostatic hypotensive reaction.23)
There were several limitations in our study. Firstly, since a valid translation of a COMPASS questionnaire in Korean version had not been established, there might be a little bias due to cultural difference. Secondly, the questions about frequency and severity of autonomic symptoms were composed of somewhat vague items. However, we provided participants with additional explanation for the questions to overcome the subjectivity in rating the severity of symptoms. Because we didn't find any significant difference of the total COMPASS scores between men and women, we didn't further analyze correlation between COMPASS and HRV domain score in any one gender group.
In conclusion, the COMPASS score correlated with some component score of the HRVin diabetics. The HRV may be used as a tool to detect diabetic autonomic neuropathy by augmentation with position change.
ACKNOWLEDGEMENTS
This work was supported by a grant from Advanced Biometric Research Center (ABRC), Republic of Korea.