|
 |
- Search
| Korean J Fam Med > Volume 33(3); 2012 > Article |
Abstract
Background
Testosterone levels are decreased in diabetic patients and recent studies have suggested that high-normal fasting glucose is a risk factor for cardiovascular disease. To further elucidate the relationship between plasma glucose and testosterone, we investigated the association between fasting plasma glucose (FPG) and endogenous sex hormones (serum total testosterone, sex hormone binding globulin, estradiol, and the ratio of testosterone to estradiol) in non-diabetic and pre-diabetic men.
Methods
This study included 388 men (age ≥ 40 years) who visited the health promotion center of a university hospital from May 2007 to August 2008. The subjects were divided into quartiles based on their FPG levels and correlation and multiple linear regression analyses were performed. Q1 (65 mg/dL ≤ FPG < 88 mg/dL), Q2 (88 mg/dL ≤ FPG < 94 mg/dL), Q3 (94 mg/dL ≤ FPG < 100 mg/dL) and Q4 (100 mg/dL ≤ FPG < 126 mg/dL).
Results
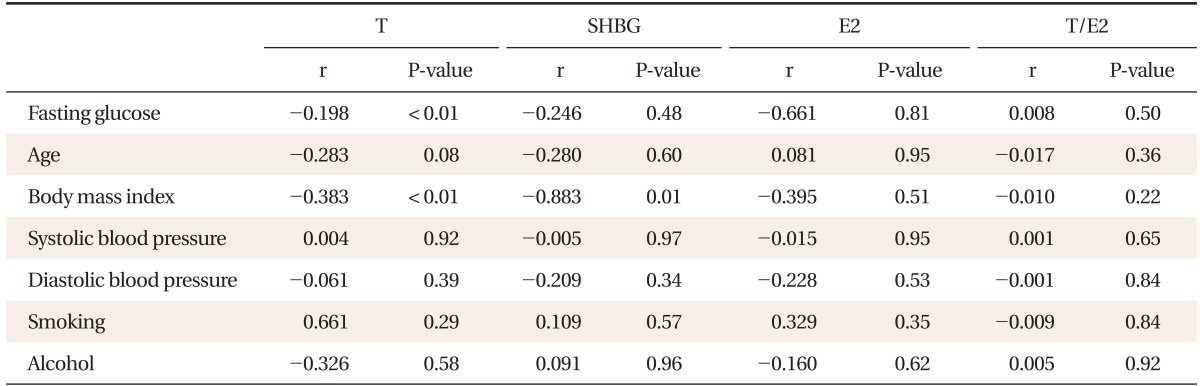
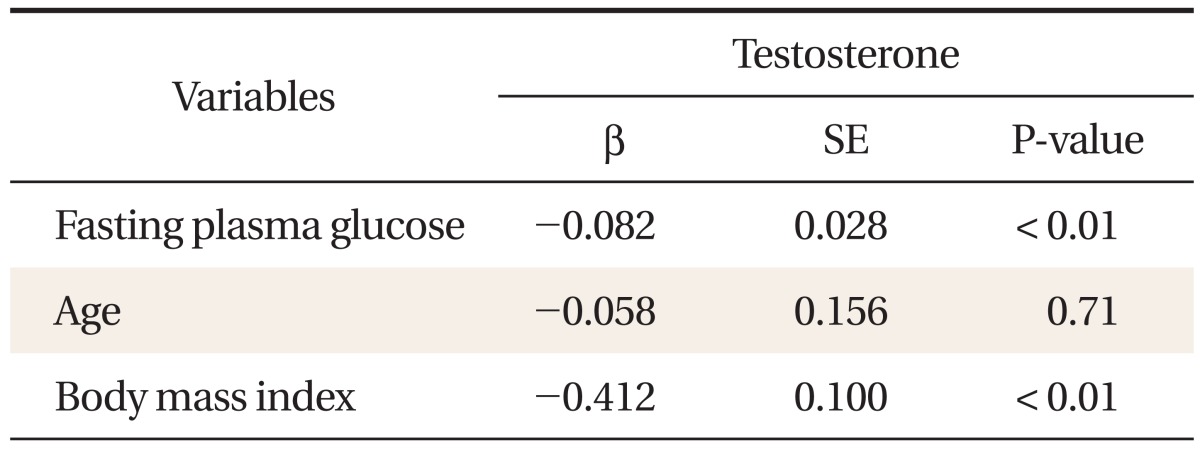
FPG was independently, inversely associated with total testosterone in the non-diabetic population after adjusting for age, body mass index, smoking, and alcohol consumption (β = -0.082, P < 0.01). Among the quartiles, subjects in the high-normal FPG groups (Q2, Q3, and Q4 with FPG ≥ 88 mg/dL) had significantly decreased testosterone levels when compared with subjects in the normal FPG group (Q1 with FPG < 88 mg/dL, P < 0.005). Sex hormone binding globulin, estradiol and the ratio of testosterone to estradiol were not correlated with FPG.
Testosterone is associated with bone mineral density, body composition, mood, aggression, cognitive function, and sexual function.1) Several studies have reported decreased testosterone levels with metabolic syndrome, visceral obesity, atherosclerosis, and type 2 diabetes mellitus (DM) in men.2-6) Studies have also suggested a relationship between fasting glucose and androgen levels in patients with diabetes, metabolic syndrome, coronary artery disease, and erectile dysfunction.7-9) Hyperinsulinemia and excess leptin may affect the suppression of sex hormone binding globulin (SHBG) activity and plasma testosterone.10,11) However, there is currently no data about these relationships in non-diabetic men with high-normal glucose levels or in pre-diabetic men.
Our previous study revealed that subjects with high-normal glucose levels were characterized by a high incidence of arterial stiffness.12) This suggests that fasting plasma glucose (FPG), even within the normal range, may affect androgen levels. Furthermore, this approach would be valuable because FPG can be easily measured in primary care settings and is a reversible risk factor. In this study we assessed the relationship between FPG, total testosterone, and SHBG as well as the testosterone to estradiol ratio in non-diabetic men with high-normal glucose levels, and in pre-diabetic men.
We recruited 430 men (age ≥ 40 years) who visited the health promotion center of a university hospital from May 2007 to August 2008. Twenty-eight subjects with a history of taking anti-diabetes medication, eight with stroke or cardiovascular disease, one with extremely low FPG levels (50-65 mg/dL), and five subjects who had previously taken testosterone and glucocorticoid medications were excluded. After these exclusions, 388 subjects were included in this study. The Institutional Review Board of Gangnam Severance Hospital, Yonsei University College of Medicine approved this study and informed consent was obtained from each participant.
According to FPG levels, subjects with normal FPG and impaired fasting glucose (IFG) levels were divided into quartiles: Q1 (65 mg/dL ≤ FPG < 88 mg/dL), Q2 (88 mg/dL ≤ FPG < 94 mg/dL), Q3 (94 mg/dL ≤ FPG < 100 mg/dL), and Q4 (100 mg/dL ≤ FPG < 126 mg/dL). Anthropometric measurements were used to calculate the body mass index (BMI). A questionnaire was used to obtain information about each subject's medical history and lifestyle, such as smoking habits and alcohol consumption. Blood pressure was measured at rest after a period of five minutes. After an overnight fast, serum glucose levels were measured using a Hitachi 7600-110 chemistry auto-analyzer (Hitachi, Tokyo, Japan). Total testosterone, SHBG, and estradiol levels were measured by electrochemiluminescence (Modular E-170; Roche Diagnostic Systems, Basel, Switzerland).
SAS ver. 9.1 (SAS Inc., Cary, NC, USA) was utilized for statistical analyses. Mean values of clinical characteristics were compared among the four subject groups using one-way analysis of variance for continuous variables and the chi-squared test for categorical variables. Pearson's correlation coefficients were calculated to evaluate the relationships between testosterone, ratio of testosterone to estradiol, SHBG, and other clinical variables. Multiple linear regression analysis was performed after adjusting for age, BMI, smoking, and alcohol consumption in order to identify any independent associations between testosterone and FPG. P-values less than 0.05 were considered statistically significant.
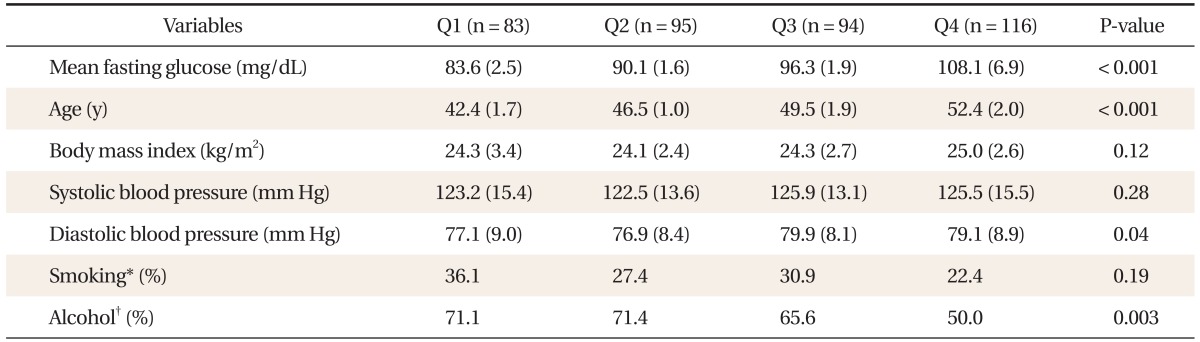
The clinical characteristics of the 388 men enrolled in this study are shown in Table 1. The mean glucose level of the IFG group (Q4) was 108.1 ± 6.9 mg/dL. The mean glucose level of normoglycemic subjects was 90.2 ± 5.7 mg/dL. Mean fasting glucose level, age, diastolic blood pressure, and alcohol consumption differed between quartiles. The mean BMI of all subjects was within the overweight range. The mean systolic blood pressure was in the pre-hypertensive stage, while the diastolic blood pressure was in the normotensive stage as defined by the Joint National Committee on Evaluation, and Treatment of High Blood Pressure guidelines.
FPG was inversely correlated with total testosterone (Table 2, Figure 1). After adjustment for age, BMI, smoking, and alcohol consumption, FPG was independently associated with total testosterone levels in the non-diabetic and pre-diabetic men (β = -0.082, P < 0.01) (Table 3). Total testosterone was significantly decreased in non-diabetic subjects with high-normal glucose and IFG levels. In particular, subjects in the high FPG groups (above 88 mg/dL) showed decreased testosterone levels after adjusting for age, BMI, smoking, and alcohol consumption (Figure 2). SHBG, estradiol, and the ratio of testosterone to estradiol were not correlated with FPG.
In this study, FPG was negatively and independently associated with total testosterone in non-diabetic Korean men. Interestingly, high-normal glucose and IFG levels were associated with a significant decrease in serum total testosterone. FPG levels can be measured easily in the outpatient clinic setting, allowing for early intervention. Therefore, we suggest that FPG levels could be an important marker for men's health risk assessment in non-diabetic patients.
In previous studies, testosterone was shown to be negatively correlated with insulin, leptin, and the homeostasis model assessment of insulin resistance.13) Hyperinsulinemia may suppress SHBG synthesis and decrease serum testosterone, thereby regulating the levels of luteinizing hormone. Studies have also suggested that hyperinsulinemia may suppress testosterone synthesis in the testis.10,11,14,15)
According to recent treatment guidelines, testosterone replacement therapy is recommended when total testosterone levels are below 8 nmol/L, while individuals with serum levels above 12 nmol/L are not categorized as having sexual dysfunction and are thus not candidates for replacement therapy. Although the cohort in this study had normal testosterone levels with no abnormal clinical signs indicating hypogonadism, glycemic levels should be carefully monitored considering the association between hypogonadal symptoms and diabetes.6,16)
In our study, estradiol levels were not correlated with FPG.8) Yasui et al.17) and Dunajska et al.18) reported a correlation between estradiol and FPG, although the subjects in their studies were Japanese and differences in demographics could account for the variation. Other lifestyle factors such as smoking were not associated with testosterone levels, and this finding was supported in a study by Halmenschlager et al.19)
Our study has some limitations with respect to extrapolating generalizations from our results. The narrow age range is useful since it suggests that men in this range should monitor their glycemic levels carefully. However, the results of this study cannot be applied to older individuals. Further research is needed to elucidate the relationship between testosterone and plasma glucose in older populations. Second, since insulin levels were not measured in our cohort, we cannot comment on the effect of insulin on testosterone metabolism. Finally, a recent study showed that up to 5% of subjects with IFG appear to have diabetes according to the 2-hour glucose tolerance test.20) Therefore, true-DM and IFG groups were not demarcated in the glucose tolerance test, implying that some individuals in the IFG group may actually have had DM.
Although this study has some limitations, we demonstrated that FPG is negatively and independently associated with total testosterone in non-diabetic, middle-aged Korean men, and may be a useful marker to control glycemic levels in order to prevent decreased testosterone levels in this population.
References
1. Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res 2001;56(Suppl 1):86-92. PMID: 11786693.


2. Blouin K, Despres JP, Couillard C, Tremblay A, Prud'homme D, Bouchard C, et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism 2005;54:1034-1040. PMID: 16092053.


3. Laaksonen DE, Kainulainen S, Rissanen A, Niskanen L. Relationships between changes in abdominal fat distribution and insulin sensitivity during a very low calorie diet in abdominally obese men and women. Nutr Metab Cardiovasc Dis 2003;13:349-356. PMID: 14979681.


4. Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis--immunomodulation and influence upon plaque development and stability. J Endocrinol 2003;178:373-380. PMID: 12967330.


5. Kalme T, Seppala M, Qiao Q, Koistinen R, Nissinen A, Harrela M, et al. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab 2005;90:1550-1556. PMID: 15613437.


6. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911-917. PMID: 17392552.


7. Zou B, Sasaki H, Kumagai S. Association between relative hypogonadism and metabolic syndrome in newly diagnosed adult male patients with impaired glucose tolerance or type 2 diabetes mellitus. Metab Syndr Relat Disord 2004;2:39-48. PMID: 18370675.


8. Han H, Shin JH, Lee CB, Park YS, Kim DS, Ahn YH, et al. The relation of the testosterone level with metabolic syndrome and coronary artery disease in Korean middle-aged and elderly men. Korean J Med 2007;73:34-43.
9. Lee SY, Kim SC. Correlation of the serum testosterone level with insulin resistance and metabolic syndrome in patients of erectile dysfunction and benign prostatic hyperplasia. Korean J Urol 2008;49:556-561.

10. Lima N, Cavaliere H, Knobel M, Halpern A, Medeiros-Neto G. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord 2000;24:1433-1437. PMID: 11126339.


11. Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol 2003;149:583-589. PMID: 14641001.


12. Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc Diabetol 2011;10:30PMID: 21492487.



13. Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl 2006;52:355-361. PMID: 16873135.


14. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415-1428. PMID: 15836891.


15. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833-876. PMID: 15901667.


16. Rice D, Brannigan RE, Campbell RK, Fine S, Jack L Jr, Nelson JB, et al. Men's health, low testosterone, and diabetes: individualized treatment and a multidisciplinary approach. Diabetes Educ 2008;34(Suppl 5):97S-112S. PMID: 19020265.


17. Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, et al. Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clin Chim Acta 2008;398:43-47. PMID: 18755173.


18. Dunajska K, Milewicz A, Szymczak J, Jedrzejuk D, Kuliczkowski W, Salomon P, et al. Evaluation of sex hormone levels and some metabolic factors in men with coronary atherosclerosis. Aging Male 2004;7:197-204. PMID: 15669538.


19. Halmenschlager G, Rossetto S, Lara GM, Rhoden EL. Evaluation of the effects of cigarette smoking on testosterone levels in adult men. J Sex Med 2009;6:1763-1772. PMID: 19473474.


20. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708-723. PMID: 12207806.


Figure 1
The relationship between fasting plasma glucose (FPG) and total testosterone. The values of total testosterone were inversely correlated with FPG in middle-aged, non-diabetic Korean men (r = -0.198, P < 0.01). There was no subject with FPG level less than 80 mg/dL.
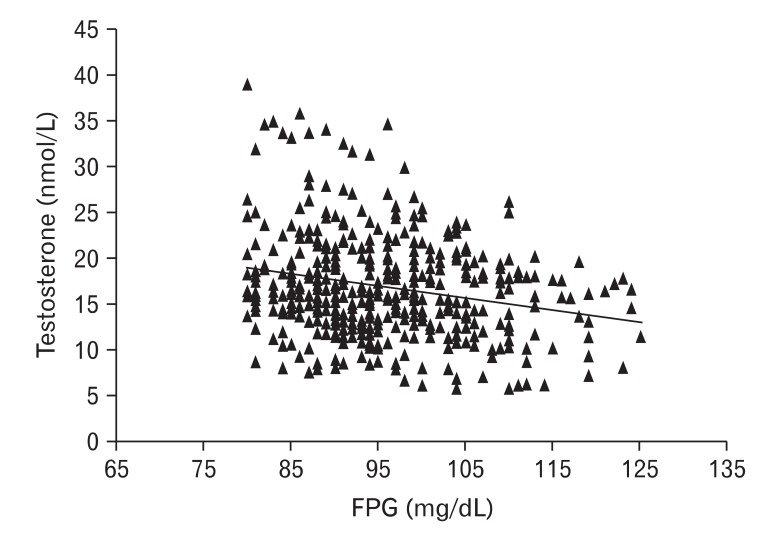
Figure 2
Adjusted mean values of total testosterone according to fasting plasma glucose (FPG) categories in middle-aged, non-diabetic Korean men. The values are mean ± SE, adjusted for age, body mass index, smoking, and alcohol consumption. P-values were calculated by analysis of covariance: Q1 (65 mg/dL ≤ FPG < 88 mg/dL), Q2 (88 mg/dL ≤ FPG < 94 mg/dL), Q3 (94 mg/dL ≤ FPG < 100 mg/dL), and Q4 (100 mg/dL ≤ FPG <126 mg/dL). *P < 0.005. †P < 0.01.
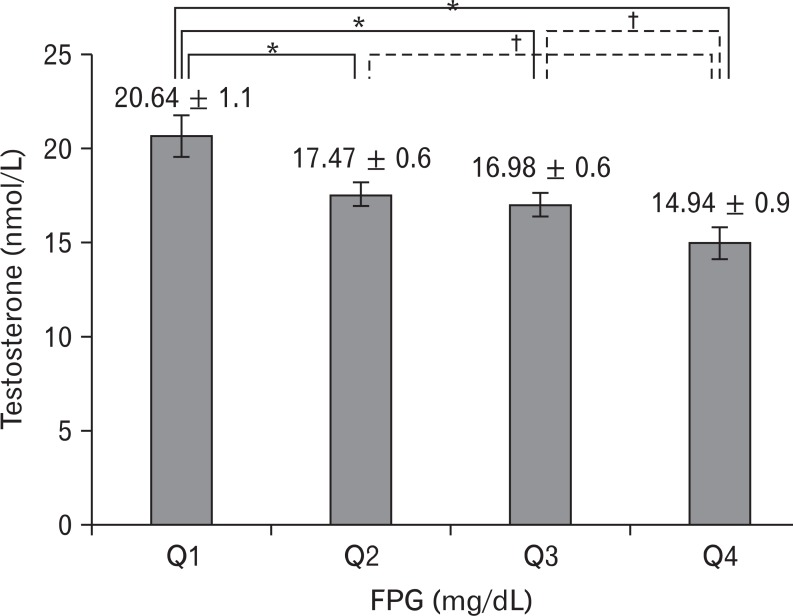
Table 1
Clinical and metabolic characteristics of study participants categorized by fasting plasma glucose into four quartiles.