Molluscum Contagiosum as a Skin Manifestation of Immune Reconstitution Inflammatory Syndrome in an AIDS Patient Who Is Receiving HAART
Article information
Abstract
Highly active antiretroviral therapy (HAART), which restores specific immune responses, may paradoxically cause an inflammatory reaction known as immune reconstitution inflammatory syndrome (IRIS). We report a patient with acquired immune deficiency syndrome, who presented Molluscum contagiosum as IRIS after HAART, the first case in Korea.
INTRODUCTION
The introduction of highly active antiretroviral therapy (HAART) has remarkably decreased the rates of opportunistic infections, the progression to acquired immune deficiency syndrome (AIDS), and the overall mortality of human immunodeficiency virus (HIV)-infected patients.1)
After initiating HAART with AIDS patients, CD4+ T lymphocyte counts rise and viral load decreases. During the initial phase of immunological reconstitution, clinical deterioration, which is a result of inflammatory reactions to several pathogens, occurs. This inflammatory reaction, which is known as immune reconstitution inflammatory syndrome (IRIS), has led to a substantial change in the spectrum of cutaneous disorders.2)
Mortality as a direct consequence of IRIS is low when the disease is limited to the skin. However, by virtue of the skin's visibility and the frequency of cutaneous IRIS, the skin provides a unique opportunity to study IRIS. Furthermore, cutaneous IRIS may serve as a visible marker for an appropriate response to HAART in resource-limited settings.3) Molluscum contagiosum (MC) is caused by a double-stranded DNA virus belonging to the pox virus family, usually seen in otherwise healthy young children. MC lesions are usually pearly, dome shaped, small, and discrete with central umbilication. In HIV-positive patients, atypical varieties are found. They may be large or non-umbilicated.4) We describe a case of AIDS and HIV-associated encephalopathy in which MC developed as a manifestation of IRIS after the initiation of HAART.
CASE REPORT
A 46-year-old, Asian, male patient was diagnosed with AIDS with pneumonia in December 2010. HIV-encephalopathy was suspected on magnetic resonance imaging diffusion imaging and JC/BK virus polymerase chain reaction on the cerebrospinal fluid revealed negative findings. The patient refused antiviral treatment and did not return for scheduled appointments for HAART after he was diagnosed.
Two months later, the patient developed a confused mental state and gait disturbance. The patient started HAART with Combivir (GlaxoSmithKline, London, UK), Reyataz (Bristol-Myers Squibb, New York, NY, USA), and Norvir (Abbott, Chicago, IL, USA). The patient's HIV viral load was 826,000 copies/mL and CD4+ count was 6.59 cells/µL at admission.
Ten days later, multiple papules which were skin-colored and dome shaped in variable sizes were present on the face and neck (Figure 1). The lesions were confirmed as MC by a dermatologist. Some lesions were surrounded by inflammation and varying size of erythema. Some evolved with central-umbilication. At this point, the CD4+ count was 20.28 cells/µL and the serum HIV viral load was 73,200 copies/mL. Some large lesions were curettaged.
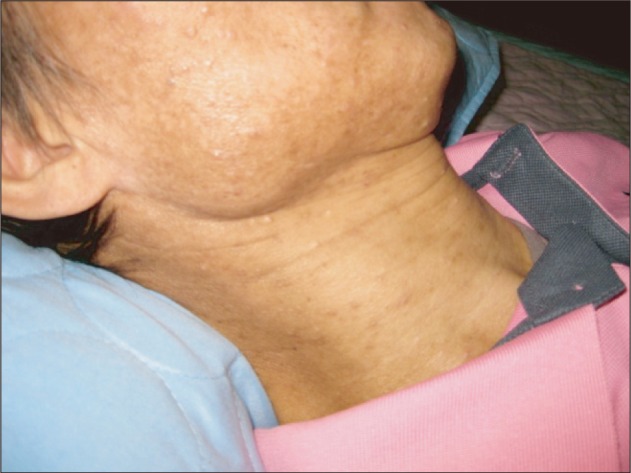
Multiple skin-colored, dome shaped and varying sized papules on the face and neck, with central umbilication in a few lesions.
In spite of increasing CD4+ lymphocyte count from 6.59 cells/µL to 20.28 cells/µL after 10 days of HAART, the CD4+ count was still under 200 cells/µL, suggesting a severely immunosuppressive state. The patient continued with HAART and was discharged. The skin lesions spontaneously healed and there were some residues.
DISCUSSION
Many researchers have suggested criteria for distinguishing IRIS from recurrence or relapse of an infection. The current definition of IRIS includes five fundamental criteria: 1) confirmed case of HIV, 2) temporal association between development of IRIS and initiation of HAART, 3) specific host responses to HAART, such as an increase in CD4+ cell count and decrease in HIV viral load, 4) clinical deterioration characterized by an inflammatory process, and 5) exclusion of other causes that may lead to a similar clinical presentation.5)
According to French et al.,6) the present patient corresponds to one of the major criteria and one of the minor criteria. HIV RNA concentration of the patient decreased to 75,280 copies/mL, and CD4+ count increased to 13.69 cells/µL after HAART. Another diagnostic criteria proposed by Shelburne et al.7) coincided with our patient in three of the criteria: HIV-positive, decrease in HIV-1 RNA level from baseline and increase in CD4+ count from baseline after receiving HAART, and clinical symptoms consistent with an inflammatory process. However, these current diagnostic criteria still have some limitations.5) In this case, the CD4+ count of the patient is increased; however, it is unclear that the degree of increase in CD4+ cell count is enough to lead to IRIS. The researchers defined the criteria including "increasing of CD4+ cell count," but there is no definite standard for the amount of increase in CD4+ cells.
There are various manifestations of IRIS. Among these clinical features, frequently reported pathogens associated with IRIS are Mycobacterium tuberculosis, atypical mycobacterium, cytomegalovirus, varicella zoster virus, and Cryptococcal neoformans. However, there are some less common pathogens including Pneumocystis jirovecii pneumonia, toxoplasmosis, hepatitis B and C virus, MC and genital warts, sinusitis, and AIDS-related lymphoma.8) In the cohort reported by Ratnam et al.,9) 4 of 199 (2%) patients experienced MC within 6 months after initiating HAART. One of 59 (1.7%) MC events were observed in another retrospective cohort study followed up 6 months after HAART initiation.10) MC is very common in HIV patients. In association with IRIS, however, cases of MC have not been reported often.2) There is a possibility of MC being under-reported as IRIS. There is no exact estimate of the incidence of MC yet.
The interval between the initiation of HAART and the beginning of IRIS is highly variable (from 1 week to more than 1 year), but in the majority of the cases, it occurs during the first two months of HAART.11) In a retrospective cohort study, the median time to the first skin IRIS diagnosis was 8 weeks (range, 3 to 24 weeks) and this is similar to that in equivalent studies.10) The clinical symptoms of MC consist of an atypical inflammatory skin process usually not found in the natural course of the disease. Those lesions, which were not mechanically removed, spontaneously resolved without any specific therapy and required only symptomatic treatments. However, the interruption in HAART therapy may exacerbate the patients' immunological status and increase the number of MC lesions.2,12,13)
In spite of the relatively short interval between initiation of HARRT and the onset of MC, we concluded this patient is a case of IRIS. Because there is a time relation between the emergence of cutaneous lesions and the reconstitution of the immune system, CD4+ cell count was increased and viral load was decreased. There was also spontaneous healing of MC lesions with the maintenance of HAART.
C-reactive protein, interferon-inducible protein 10 or interferon γ were suggested as predictors of IRIS events.14) Statins, in addition to their lipid-lowering effects, have anti-inflammatory attributes and there is precedence for the use of these agents as a therapeutic modality for autoimmune inflammatory disorders which have similar underlying pathogenesis as immune reconstitution inflammatory syndrome.15) A case definition of IRIS is still not clarified because little is known about IRIS at this time, and IRIS is likely to become a serious problem in the next decade. Therefore, large prospective studies are needed to elucidate the predictive and diagnostic value of biomarkers, treatment, and diagnostic criteria of IRIS.
Notes
No potential conflict of interest relevant to this article was reported.