Association between Metabolic Components and Subclinical Atherosclerosis in Korean Adults
Article information
Abstract
Background
Many studies have attempted to develop relatively simple and easy noninvasive measurements of atherosclerosis (NIMA), and each NIMA assesses different atherosclerotic properties. We, therefore, investigated the association between metabolic syndrome (MetS) components and different NIMAs.
Methods
This study included 1,132 Korean subjects over 20 years of age who had visited a Health Promotion Center in Korea. Carotid injury (increased carotid intima-media thickness or plaques) was evaluated by ultrasonography and arterial stiffness by brachial-ankle pulse wave velocity. The MetS components were assessed according to the Asian criteria of the American Heart Association/National Heart, Lung, and Blood Institute.
Results
Both arterial stiffness and carotid injury gradually deteriorated with increase in the number of MetS components. Arterial stiffness and carotid injury were associated with different MetS components, each of which had varying impact. After adjustment for all possible confounders such as age, sex, and lifestyle, elevated blood pressure (BP) was found to have the strongest association with arterial stiffness, whereas central obesity, impaired fasting plasma glucose, and elevated BP had comparable connection with carotid atherosclerosis.
Conclusion
Individual MetS components were related with subclinical atherosclerosis in different ways. Elevated BP showed the strongest association with arterial stiffness, while central obesity, impaired fasting plasma glucose, and elevated BP showed good correlation with carotid atherosclerosis.
INTRODUCTION
The question of why some individuals develop early cardiovascular disease (CVD) and others do not despite the presence of traditional risk factors has initiated new research on atherosclerosis.1) This contradiction is potentially due to genetic susceptibility, combinations and interactions between risk factors, and the duration of exposure to specific levels of these risk factors.2) Thus, subclinical disease measurements, which represent the final result of risk exposure and genetic susceptibility, may be useful to improve risk stratification, therapeutic strategies, and evaluation of risk factor modification in CVD.
Several noninvasive measurements of atherosclerosis (NIMA) have been developed over the past few years to identify and quantify accelerated atherosclerosis. They are primarily used for risk stratification of individuals in order to predict CVD and in the short-term effectiveness of CVD treatment. These methods facilitate the assessment of early atherosclerotic changes such as endothelial dysfunction, arterial stiffening, and measures of arterial remodeling, which are considered surrogates of CVD and atherosclerosis.3,4)
Atherosclerosis represents the process of arterial aging that leads to diminished elastic properties of the medial layers and increased stiffness; in addition, it represents a chronic inflammatory process that causes specific thickening of the arterial wall. Currently, some NIMA assess arterial stiffness (e.g., pulse wave velocity [PWV]) and reflect the structural deterioration of the arterial wall (e.g., carotid intima-media thickness [CIMT] or carotid plaques). PWV and CIMT are easy to obtain, require less training prior to application, and their results are highly reproducible; therefore, these methods have considerable research backing and clinical utility and the associated equipment is readily available for use.5)
Metabolic syndrome (MetS) is the occurrence of conventional CVD risk factors as a cluster in one person, and its diagnosis appears to be a better predictor of CVD risk than individual risk factors taken together.6) Thus far, some association has been reported between MetS or its components and any one specific NIMA in a selected group; however, there is relatively little data linking individual MetS components with different NIMA in the same group of subjects. Determining such association may help us identify individuals who are at a high risk for subclinical atherosclerosis, and to explain discordant results of NIMA to the patients. Therefore, in this study, we investigated the correlations between specific MetS components and the two most commonly used NIMA in a sample of the general population.
METHODS
1. Subjects
The present study included 24,843 adults over 20 years of age who visited the Health Promotion Center of Dongguk University Ilsan Hospital in Goyang, Korea, between June 2007 and December 2010 for annual checkups. The analysis for the present study was limited to 1,201 subjects who underwent brachial-ankle pulse wave velocity (baPWV) and carotid ultrasound voluntarily, whose medical records were retrospectively reviewed. Subjects with overt CVD, established renal disease (serum creatinine > 1.4 mg/dL in females and > 1.5 mg/dL in males), any liver diseases (aspartate aminotranserase or alanine aminotranserase ≥ 3 times the upper limit of the normal in our laboratory) and systemic disease such as hyper- or hypothyroidism were excluded from the study.7) Eventually, a total of 1,132 subjects were included in the final analysis of this study. The study protocol was approved by the institutional review board (IRB) of Dongguk University Ilsan Hospital, Goyang, Korea. The requirement of informed consent from the study participants was waived by the IRB.
2. Clinical Examination and Blood Assays
All medical examinations were performed by trained personnel according to standardized procedure. Data on health habits inventory and medical history were obtained through a questionnaire survey. The questions related to alcohol intake were as follows: type of alcoholic beverage consumed, frequency of alcohol consumption on a weekly basis, and amount of alcohol consumed daily. With regard to smoking status, participants were classified as non-smokers or current smokers. The participants were also queried about the type and frequency of physical activity that they engaged in on a weekly basis. A regular exerciser was defined as a person who engaged in physical activities for more than 20 minutes per session at least 3 times per week. The height and weight of the participants were measured using an automatic digital stadiometer (GL-150; G-Tech International Co., Uijeongbu, Korea), and the measurements were carried out with the subjects dressed in a light gown and standing barefoot. Body mass index was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured using non-stretchable standard tape in accordance with the World Health Organization protocol.8)
Blood samples were obtained after the participants had fasted overnight. The serum triglyceride, high-density lipoprotein-cholesterol (HDL-C), and fasting plasma glucose levels were determined using standard automated bioassays (Modular DPE; Roche Diagnostics, Mannheim, Germany).
3. Measurement of Carotid Injury and Arterial Stiffness
The same trained sonographer ultrasonically evaluated the common carotid artery (CCA) using an 8 MHz linear array transducer (Sequoia 512; Acuson, Mountain View, CA, USA) after the patient had been resting in the supine position for 15 minutes. CIMT was measured using automatic intima-media thickness (IMT) measurement software (M'Ath-Std; Metris, Argenteuil, France) on a computer. The IMT was measured 2 cm proximal to the carotid bifurcation along at least 1 cm of axial length, and then an automated edge detection algorithm measured the IMT as the distance between the lumen-intima interface and the media-adventitia interface. The mean values of the right and left CCA IMTs were used for the analysis. A plaque was defined as a localized protrusion into the vessel lumen with thickening of the vessel wall of > 50% compared to the adjacent IMT. Carotid injury was considered present when the mean CIMT was greater than 0.9 mm, or when arteriosclerotic plaques were detected.9)
Arterial stiffness was assessed by measuring the baPWV using an automated waveform analyzer (Colin VP-2000; Colin Medical Instrument Co., Komaki, Japan). This instrument can simultaneously record the profiles of several variables such as blood pressure (BP) parameters, heart rate, and ankle-brachial index. The mean baPWV measured bilaterally was used for the analysis. In a previous study in the same institute carried out with the same equipment, Pearson's correlation coefficients for intra- and inter-observer reproducibility were 0.976 and 0.972, respectively.10) The presence of arterial stiffness was defined as baPWV > 12 m/sec, based on the guidelines for cardiovascular screening in the asymptomatic at-risk population.11)
4. MetS and Its Components
MetS components were assessed according to the Asia criteria of the American Heart Association/National Heart, Lung, and Blood Institute.12) The WC cutoff was applied based on the Korean Society of the Study of Obesity.13) Therefore, MetS was defined by the presence of 3 or more of the following 5 characteristics: 1) central obesity with WC ≥ 90 cm for men and WC ≥ 85 cm for women, 2) hypertriglyceridemia with triglyceride levels ≥ 150 mg/dL, 3) low HDL-C with HDL-C < 40 mg/dL for men and < 50 mg/dL for women, 4) elevated BP, with BP ≥ 130/85 mm Hg or receiving antihypertensive medication, and 5) elevated fasting plasma glucose (FPG) level (FPG ≥ 100 mg/dL) or receiving antidiabetic medication (insulin or oral agents).
5. Statistical Analysis
Descriptive statistics were presented as number (percentage) for categorical variables and as mean values ± SD for continuous data. Trends of mean or frequency among groups according to the number of MetS components were estimated using a linear regression model or a linear by linear association test. To express the impact of each MetS component on arterial stiffness or carotid injury, the odds ratios (ORs) with 95% confidence interval (CI) were calculated after adjusting for confounding variables by using multivariate logistic regression models. All statistical analyses were performed using the SPSS ver. 16.02 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
According to the definition of the modified National Cholesterol Education Program criteria, the prevalence of MetS was 25.0% (26.5% and 19.6% for male and female subjects, respectively). The patient characteristics according to the number of MetS components are presented in Table 1. There was a significant increase in the age, body mass index, percentage of men, and problem drinkers with increase in the number of MetS components. Both arterial stiffness and carotid injury gradually increased according to the number of MetS components, and it is noteworthy that subjects with only 1 or 2 traits already showed more associations with subclinical atherosclerosis. This trend was significantly apparent even after adjustment for confounders such as age, sex, body mass index, smoking status, alcohol amount, and regular exercise (data not shown).
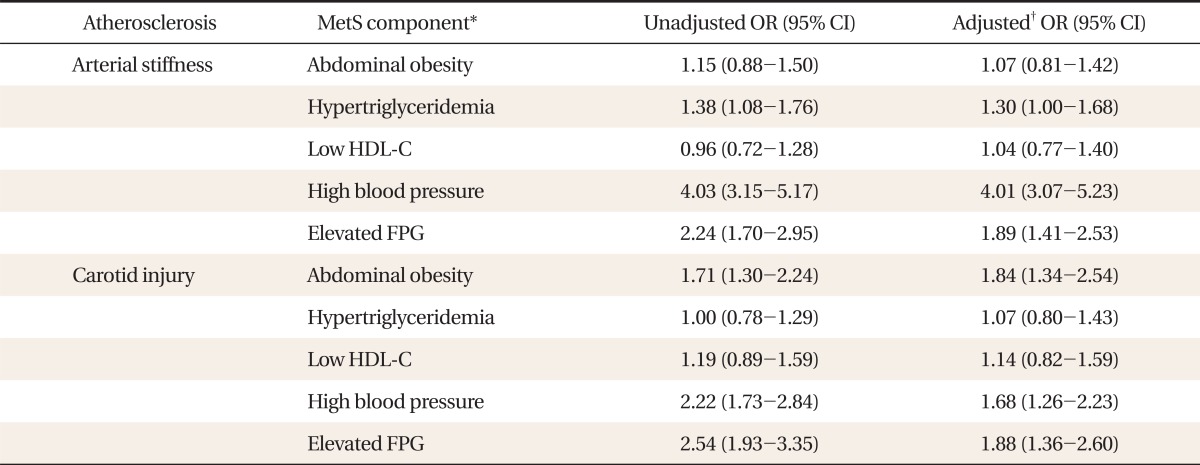
Table 2 shows the association between metabolic components and subclinical atherosclerosis. High BP and elevated FPG were related significantly with abnormality of both subclinical atherosclerosis, and abdominal obesity was an additional significant associated factor with carotid injury alone. In arterial stiffness, only one factor (high BP) had a prominent association (OR, 4.01; 95% CI, 3.07 to 5.23). Meanwhile, except for the lipid profile, the remaining 3 factors yielded comparable estimates of carotid injury.
Figure 1 shows the relationship between individual components with outcomes of 2 NIMA. The findings were consistent after adjustment of other MetS components, confirming the results presented in Table 2. High BP and elevated FPG were associated with arterial stiffness, with a remarkable association particularly between high BP and stiffness. Abdominal obesity was found to be another significant connected factor with carotid injury, and the associations of the 3 components with carotid injury were similar.

Association with metabolic components and subclinical atherosclerosis. Carotid injury was defined as the presence of plaques or carotid-intima medial thickness as measured by ultrasonography of ≥0.9 mm, and arterial stiffness in terms of brachial ankle pulse wave velocity of >12 m/sec. Metabolic components were based on the diagnostic criteria of American Heart Association/National Heart, Lung, and Blood Institute & waist circumference (Korean women): waist circumference ≥90 cm for men and ≥85 cm for women; triglyceride level ≥150 mg/dL, high density lipoprotein cholesterol (HDL-C) of <40 mg/dL for men and <50 mg/dL for women, blood pressure ≥130/85 mm Hg or receiving antihypertensive drugs; fasting plasma glucose of ≥100 mg/dL or receiving antidiabetic medication. The odds ratio (OR) and 95% confidence intervals were extracted after adjustment for age, sex, smoking status, regular exercise, alcohol consumption, and 4 additional components. FPG: fasting plasma glucose.
DISCUSSION
This study showed that arterial stiffness and carotid injury were associated with different MetS components and that each component had varying relationships with the two NIMA. Elevated BP was the most prominent component associated with arterial stiffness, while elevated BP, impaired FPG, and central obesity had similar association with carotid atherosclerosis.
Until now, a few studies have focused on the association between MetS components and different NIMAs in the general population.14) Similar to this study, Czernichow et al.14) used PWV and carotid ultrasonography in their study and reported that elevated BP was the only MetS component associated with both vascular parameters. Their results are in overall accordance with ours, with a few differences. First, the cutoff value for elevated FPG in their study was 110 mg/dL, which may have curtailed the prevalence of impaired FPG. The prevalence of each component may be responsible in part for the predominant effect reflected on vascular parameters. Second, in contrast with our analysis of the components, which was dichotomous, the previous study used a linear regression model with MetS components as continuous variables, which is less effective for distinguishing notable differences. Furthermore, their assessment of carotid injury was divided into 2 categories-increased CIMT and the presence of plaques. Although measuring the thickness and detecting plaques probably reflect different aspects in the development of atherosclerosis, the predictive values of each and both these parameters are still debated and vary across studies.15) In clinical practice, the fact that both measurements are derived from the same instrument is significant.
Interestingly, central obesity was associated with carotid injury but not with arterial stiffness. Obesity itself may be directly related to atherosclerosis possibly through increased inflammatory markers, which are mostly associated with regional rather than general adiposity. It may also lead to endothelial dysfunction through the development of hypertension, diabetes, and other early metabolic abnormalities such as insulin resistance and dyslipidemia.16) Several cross-sectional studies have already demonstrated that regional adiposity is related to the burden and progression of carotid atherosclerosis,17,18) although the associations with arterial stiffness are inconsistent.19) Pathophysiological processes linking abdominal adiposity to arterial stiffness are still incompletely defined. Khan et al.20) recently demonstrated significantly higher CIMT and aortic PWV values in metabolically benign overweight/obese women than in women with normal weight after multivariable adjustment. However, they focused on obesity as an independent factor for subclinical CVD, and their analysis of the linear regression model did not adjust for confounding components, despite significant differences noted in the subjects' general characteristics. Given that PWV heavily depends on BP, it is reasonable to anticipate that the significance in aortic PWV would be attenuated after adjusting for several MetS components, especially elevated BP.
The 2 NIMA methods used in our current study, namely, carotid ultrasonography and PWV, supply complementary information and should not be used interchangeably,21) and it is implicit that measuring both these traits provides better characterization of atherosclerosis. Oren et al.22) conducted a large cross-sectional study with healthy young subjects and reported that the correlation between aortic PWV and CIMT was attenuated after adjustment for clinical variables, suggesting that these 2 indices reflect different aspects of vascular damage. In addition, Tzortzis et al.23) adjusted for several confounders in never-treated hypertensive patients and demonstrated that increased CIMT and arterial stiffness are independent and complementary determinants of an impaired coronary flow reserve. Together, all these findings suggest that the combination of vascular markers that reflect functional and/or structural changes of the arterial wall has greater value for the prediction of CVD than each index alone.
We observed that even 1 or 2 MetS components results in deteriorations of NIMA, particularly of baPWV. This may be due to the independent presence of these risk factors rather than any special feature of the risks clustering in the MetS that is associated with increased arterial stiffness or carotid injury. We believe that this information is important in clinical practice because it conveys that it is more important to identify the separate MetS components than to merely identify their presence or absence, and because it also supports the notion that the components of MetS may be most potent when presented in combination. The results of the study by Holewijn et al.24) are in agreement with our results that it is more important to consider the presence of each individual trait and the number of traits than to merely diagnose the presence of the MetS. Recent studies have criticized the concept of MetS, and its clinical role is still debated.25) Therefore, in response to the uncertainty regarding MetS, clinicians should evaluate and treat all factors without regard to whether a patient meets the criteria for diagnosis of the MetS.
This study has several limitations. The first limitation of our study is its observational design, which does not enable us to draw conclusions in terms of causal relationship. The relationship between subclinical atherosclerosis and metabolic risk factors is likely to be bidirectional and interrelated2,26); therefore, further prospective studies are necessary. Second, we used baPWV instead of carotid-femoral PWV (cfPWV) to assess arterial stiffness. Although cfPWV is considered the gold standard for the measurement of arterial stiffness in Western countries,27) no single methodology is proven to be superior. Tanaka et al.28) recently reported that the cfPWV and baPWV indices of arterial stiffness are similarly associated with CVD risk factors and clinical events. Third, the generalization of our results should be taken with caution. Since the study subjects had visited a health promotion center, they might be more health conscious and affluent than the general population usually represented in community-based studies. Additionally, the current analysis was limited to the subjects who underwent subclinical atherosclerosis test voluntarily, and selection bias could exist. Fourth, we did not test intraobserver variability between the radiologists who performed carotid ultrasonography. Finally, we were able to obtain data on the effect of a lipid-lowering agent, which could have altered the lipid profile of our subjects. However, we believe that it underestimates the metabolic effect on carotid injury, as published literature suggests that statins exert beneficial effects on carotid atherosclerosis progression rather than on arterial stiffness reduction.29)
In conclusion, elevated BP was the most significant component for arterial stiffness, whereas all other components, except for lipid profiles, reflected similar degrees of association with carotid injury. This aids in understanding the clinical relevance of discordant NIMA results and identifying individuals who are at a high risk of subclinical atherosclerosis.
Notes
No potential conflict of interest relevant to this article was reported.