The Association between Oxidative Stress and Metabolic Syndrome in Adults
Article information
Abstract
Background
In this Study, we investigated the effects of lifestyle and metabolic syndrome on free oxygen radical levels in men and women in Korea.
Methods
A total of 254 adults were included in this study from February 2011 to June 2012 at a health promotion center. Information of the lifestyles and presence of metabolic syndrome factors was obtained. Biochemical markers were measured and free oxygen radicals test (FORT) was performed on the blood.
Results
Of the 254 subjects, 86 (33.9%) had metabolic syndrome, and 187 (73.6%) were men. Between the subjects with and without metabolic syndrome, there was a significant increase in alanine aminotransferase and serum FORT values in the subjects with metabolic syndrome. Multiple linear regression analysis showed that high-sensitivity C-reactive protein (hs-CRP) (P = 0.004), metabolic syndrome (P = 0.037), and female gender (P = 0.030) were independent predictors of serum FORT values. The subjects with high fasting blood sugar level or low high density lipoprotein cholesterol levels showed high serum FORT values.
Conclusion
High hs-CRP, the presence of metabolic syndrome, and female gender were associated with the high oxidative stress. High oxidative stress was associated with the presence of metabolic syndrome.
INTRODUCTION
The prevalence of metabolic syndrome has increased due to underactivity and overeating. The Korean National Health and Nutrition Examination Survey 2007-2010 reported that the prevalence rates of metabolic syndrome are 31.9% in Korean men and 25.6% in Korean women.1) Metabolic syndrome is associated with the development of cardiovascular disease, thus, the cardiovascular mortality in metabolic syndrome patients is 2 to 3 times higher than that of the general population.2,3) Oxidative stress, on the other hand, is an imbalance which occurs in the form of overproduced reactive oxygen or a shortage of antioxidants, and is associated with the development of metabolic syndrome.4)
Almost all metabolic syndrome patients suffer from abdominal obesity. An excessive accumulation of fat leads to enhanced production of oxidative stress. Increased oxidative stress in abdominal adipocytes may yield various pro-inflammatory adipocytokines and fatty acids.5) Thus, oxidative stress exacerbates metabolic syndrome factors such as insulin resistance, hypertriglyceridemia, decrement of high density lipoprotein (HDL) cholesterol, and hypertension.6-8)
However, there have been domestic studies on the relationship between oxidative stress and metabolic syndrome. Therefore, this study examined the relationship between factors of metabolic syndrome and oxidative stress in the Korean adult population. This study also examined whether metabolic syndrome makes a difference in oxidative stress values.
METHODS
This study was performed in accordance with ethical and safety guidelines upon the approval of the institutional review board in The Catholic University of Korea St. Vincent's Hospital (VC12 RISI0080).
1. Study Population
A total of 254 adults (control group 168 and metabolic syndrome group 86) participated in this study at a health promotion center located in Gyeonggi province, from February 2011 to June 2012.
2. Methods
1) Lifestyle investigation
This study used self-report questionnaires and interviews with healthcare providers to assess lifestyle, disease history, medication history, alcohol and smoking history, amount and frequency of exercise, eating habits, emotional stress, pollution exposure, and electromagnetic exposure.
2) Anthropometrics
Body weight and height was measured when standing barefoot, up to 0.1 kg and 0.1 cm, respectively. Waist circumference was measured in standing position, expiratory state. Waist circumference was measured on the middle area between the lower rib margin and the upper illiac bone margin.9) Blood pressure was measured using an automatic sphygmomanometer while sitting.
3) Biochemical analysis
Blood samples were collected after at least 8 hours of fasting to examine the total amount of cholesterol, HDL, triglycerides, low density lipoprotein (LDL), high-sensitivity C-reactive protein (hs-CRP), insulin, total protein, albumin, aspartate transaminase, alanine transaminase (ALT), gamma-glutamyl transpeptidase, blood urea nitrogen, creatinine, uric acid, glycated hemoglobin, lipoprotein A, thyroid stimulating hormone, free T4, total number of white blood cells, hemoglobin, and hematocrit.
Free oxygen radicals (FORT) was evaluated by using the FORT test (FORM Plus; free oxygen radicals monitor, Callegri, Italy). Peripheral blood samples were collected from the tip of a finger and assessed to measure the FORT values by assessing hydrogen peroxide levels. Analyzed results were presented with FORT units. One FORT unit is equal to 0.26 mg/L H2O2. Reliability, reproduction and validity of FORT have been approved in many studies. In this way, the test has been used to analyze oxidative stress levels widely.10-12)
4) Definition of metabolic syndrome
Metabolic syndrome as defined by the National Cholesterol Education Program,13) is characterized by at least three of the following: (1) Abdominal obesity, waist circumference ≥ 90 cm (in men), ≥ 85 cm (in women); (2) serum triglyceride ≥ 150 mg/dL (1.69 mmol/L); (3) serum HDL cholesterol < 40 mg/dL (1.04 mmol/L) (in men), < 50 mg/dL (1.29 mmol/L) (in women); (4) blood pressure ≥ 130/85 mm Hg or medication; (5) fasting plasma glucose ≥ 100 mg/dL (6.1 mmol/L) or medication. Abdominal obesity is defined by the reference, redefining obesity in Asia-Pacific.14)
5) Statistical analysis
Data were analyzed by using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance and t-test were used to examine the relationships between FORT and lifestyle, eating habits and presence of disease of the subjects. Pearson's correlation analyses were used to examine the relationships between biochemical markers and FORT. Multiple linear regression analysis was used to analyze the effects of various independent variables on the FORT. P-values were considered statistically significant if they were less than 0.05.
RESULTS
1. Demographic and Clinical Characteristics of the Subjects
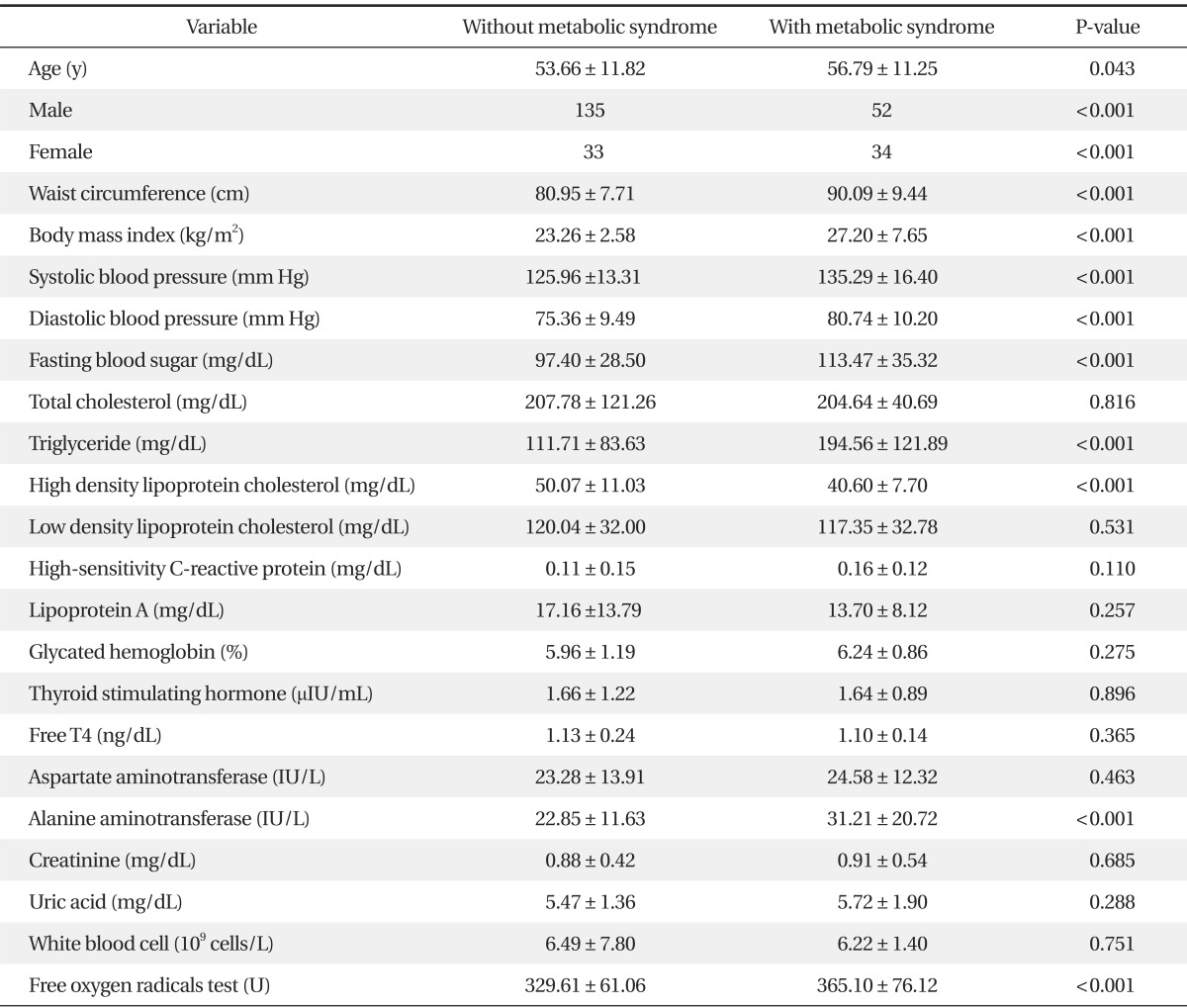
The total number of subjects was 254. There were 86 metabolic syndrome patients (33.9%) and 168 healthy controls (66.1%). There were 187 males (73.6%). ALT levels were significantly higher in the metabolic syndrome group (P < 0.05), however there was no significant difference in other factors between the metabolic syndrome group and the control group, except metabolic syndrome diagnosis factors.
The FORT values were 365.10 ± 76.12 FORT units in the metabolic syndrome group, and 329.61 ± 61.06 FORT units in the control group. A significantly higher FORT value was shown in the metabolic syndrome group (P < 0.001) (Table 1).
2. Relationship between Gender and Exogenous Factors of the Subjects and Levels of ROS Measured by the FORT
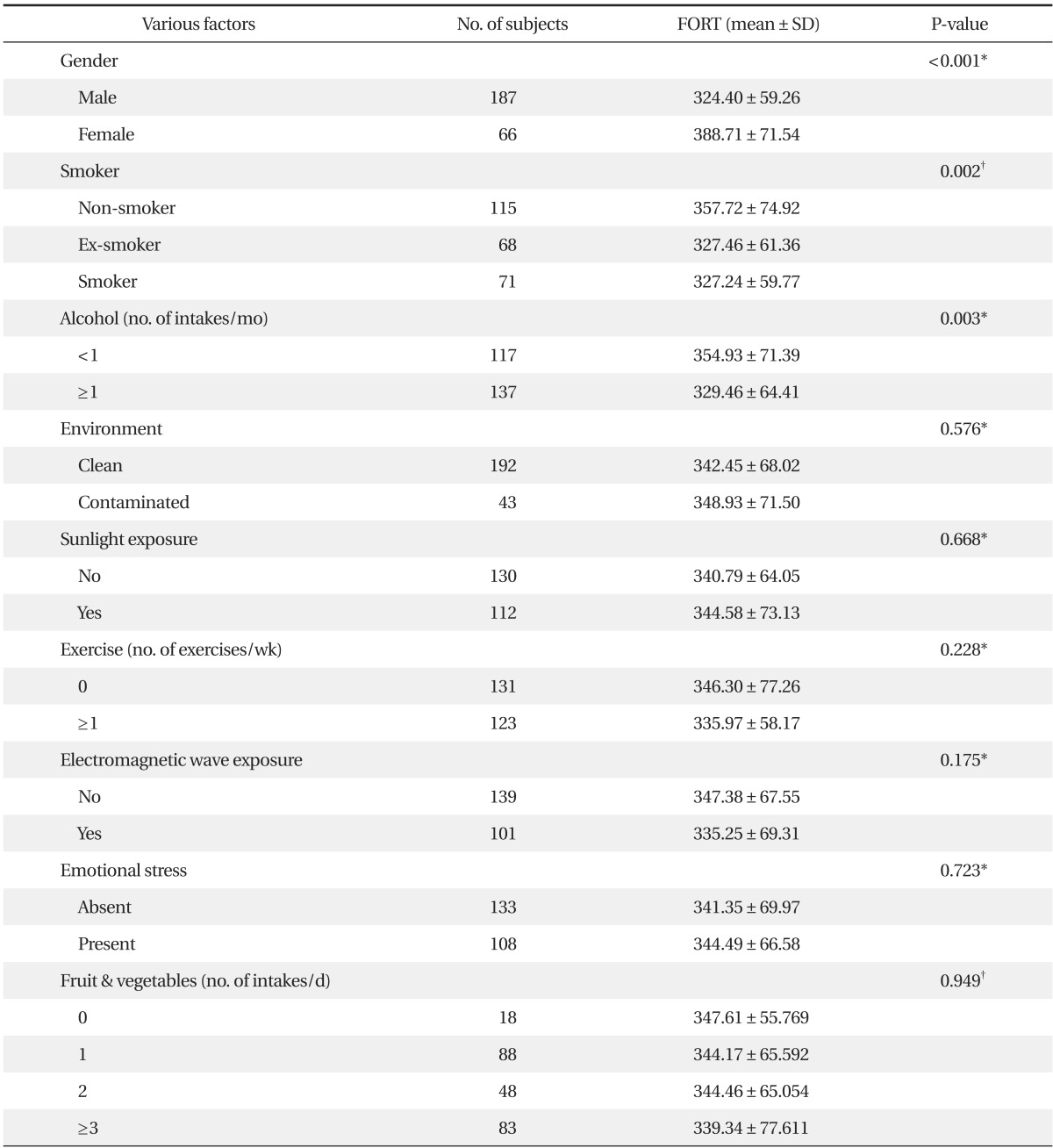
The FORT values in the non-smoking group were 357.72 ± 74.92 FORT units, in the past smoking group were 327.46 ± 61.36 FORT units, and in the present smoking group were 327.24 ± 59.77 FORT units. A significantly higher FORT value was shown in the past smoking group (P = 0.002). The FORT value in the group that consumed alcohol no more than once a month was significantly higher than the FORT value in the group consuming alcohol once or more a month (P = 0.003). Males showed a significantly higher FORT value than females (P < 0.001). However, the FORT values showed no significant difference when compared with other exogenous factors such as pollution exposure, sun exposure, amount and frequency of exercise, electromagnetic exposure, emotional stress, or eating habits (Table 2).
3. Relationship between Biological Factors of the Subjects and ROS Levels Measured by the FORT
The FORT value showed a positive correlation with hs-CRP (P < 0.001). In contrast, the FORT value showed a negative correlation with uric acid (P = 0.013). No other biochemical markers showed a statistically significant relationship with the FORT values (Table 3).
4. Analysis of the Effects of Various Independent Variables on the Free Oxygen Radical Test Values
Multiple linear regression analysis was performed to analyze the effects of statistically significant variables between the metabolic syndrome group and the control group in univariate analysis. The analysis showed hs-CRP (P = 0.004), metabolic syndrome (P = 0.037), and gender (P = 0.030) were an independent predictor of FORT values (Table 4).
5. Relationship between Metabolic Disease Factors of the Study Population and ROS Levels Measured by the FORT
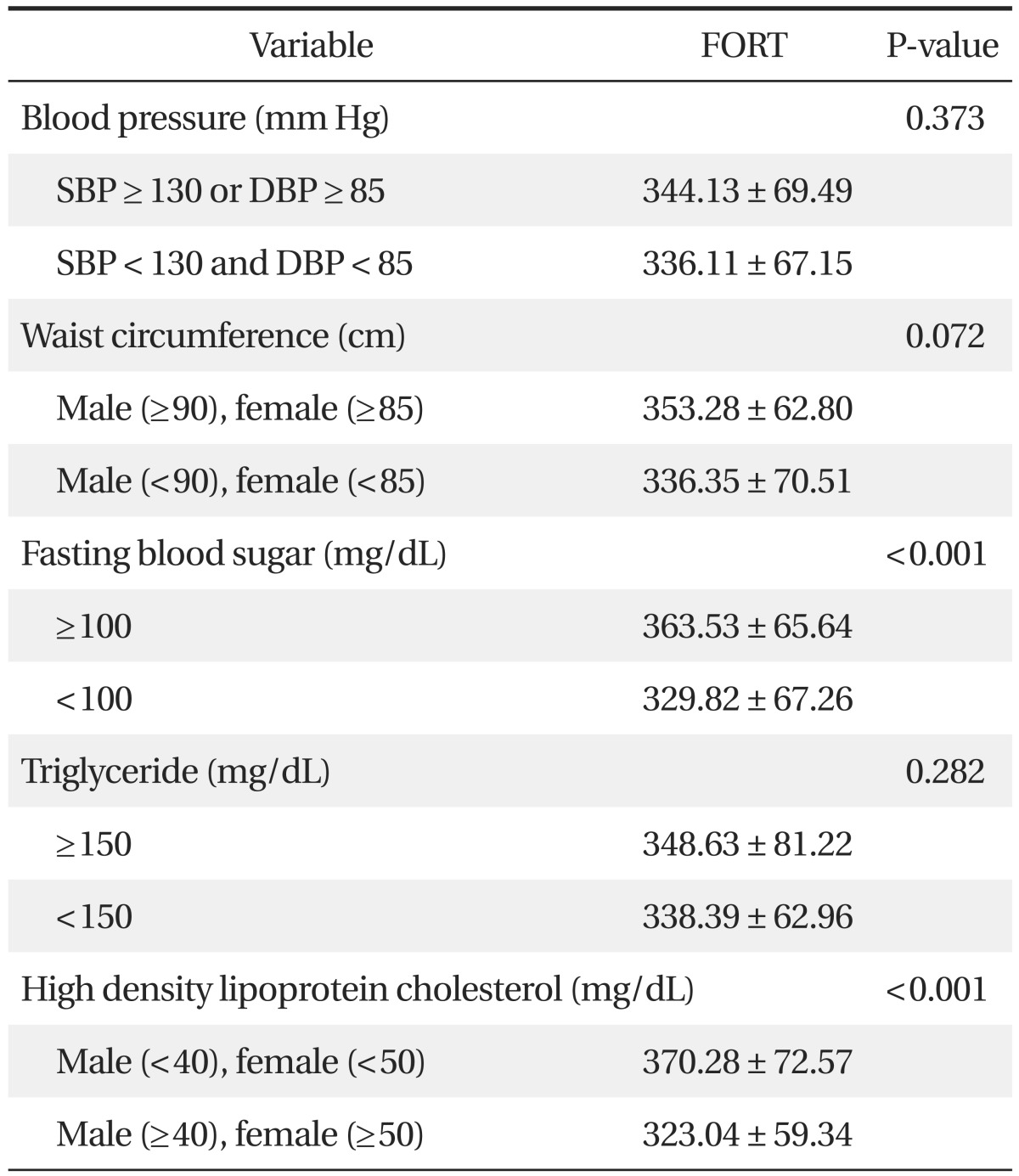
The FORT values in the subjects who had higher fasting plasma glucose or lower HDL cholesterol were significantly higher (P < 0.001). However, according to blood pressure level, waist circumference, and triglyceride level, there were no significant differences in the FORT value (Table 5).
DISCUSSION
Oxidative stress damages DNA and proteins by inducing oxidation reactions, contributing to aging, atherosclerosis, autoimmune disease, Parkinson's disease, Alzheimer's dementia, cancer, and cardiovascular disease. Overproduction and reduction of antioxidant capacity is determined by various lifestyle factors, especially non-smoking, vitamin and mineral supplements, fruit eating, and regular exercise. Male gender and aging showed negative correlations with antioxidant capacity.15)
In this study, the FORT values were significantly higher in the non-smoking group, non-drinking group, and females. The FORT value showed positive correlation with hs-CRP, and showed negative correlation with uric acid. Multiple linear regression analysis was performed to analyze the effects of these statistically significant variables. As a result, alcohol, smoking, and uric acid showed no significant effects. In contrast, female gender, higher hs-CRP, and metabolic syndrome showed significant relationships with higher FORT values.
However, when other variables were controlled through regression analysis, there was no significant relationship between smoking habits with FORT. Other studies such as Lorgis et al.16) and Lee et al.17) reported similar results. But Moller et al.18) reported smoking was associated with higher oxidative stress, Vassalle et al.19) reported females and smokers showed a significant relationship with increment of oxidative stress. In addition, Lesgards et al.15) reported that non-smokers showed a positive relationship with antioxidant capacity.
Since tobacco has many oxidating agents such as nicotine, FORT and other oxidating agents, it can contribute to atherosclerosis.20) However, many studies reported varied results. Thus, additional studies are necessary to test the relationship under control.
The FORT values showed a positive correlation with hs-CRP (P ≤ 0.001). In contrast, they showed a negative correlation with uric acid (P = 0.013). However, other biochemical markers showed no significant relationship with the FORT values. Only hs-CRP was truly related to the FORT value when the analyses of the effects of various independent variables on the FORT values were examined. Hs-CRP is an inflammatory marker and it has been examined in many studies. It has been considered as a potential marker of risk of cardiovascular disease.21,22)
Other studies reported oxidative stress as an oxidative stress marker,23-25) and Lee et al.17) reported that hs-CRP exhibited a positive correlation with the FORT value. Many other studies reported a relationship between hs-CRP and oxidative stress under controlled environments, thus, utilization of hs-CRP as an oxidative stress predictable factor could be taken into consideration. Fujita et al.26) reported that systemic oxidative stress, as measured by urinary 8-epi-PGF2, is strongly associated with visceral fat accumulation and metabolic syndrome. Kotani and Taniguchi27) reported that subjects with metabolic syndrome (n = 37) exhibited significantly higher reactive oxygen metabolites levels than those without metabolic syndrome (n = 37) in Japanese males aged under 60 years. Jialal et al.28) reported that nascent metabolic syndrome was associated with increased oxidative stress as demonstrated by both circulating and cellular biomarkers, which could contribute to the risk for both diabetes and cardiovascular disease. Similarly, in this study, metabolic syndrome patients exhibited significantly higher FORT values under controlled environments. In particular, higher fasting plasma glucose and higher LDL cholesterol levels exhibited significant relationships with the FORT values. Reactive oxygen species regenerate endogenous antioxidants, interrupt glucose absorption in muscles and fat cells, decrease the secretion of insulin in beta cells, and increase insulin resistance and fasting plasma glucose levels.29) Atherosclerosis and cardiomyopathy in type 2 diabetes are caused by insulin resistance.30) In addition, low HDL cholesterol levels are an important risk factor for cardiovascular disease. HDL cholesterol has many cardiovascular protection effects, such as anti-inflammatory effects, anti-oxidant capacity, anti-coagulation, and anti-thrombotic capacity.31)
Almost all females answered that they were non-smokers in self-reporting questionnaires. However, there was a report that only 7.1% of female subjects reported they are smokers but actually 13.9% of female subjects were proven to be smokers by examination of nicotine in their urine.32) According to this, self-reporting questionnaires on smoking habits have low reliability. Therefore, collection of urine to check nicotine levels or examination of expiratory CO2 levels is one possible solution.
Second, in women, oxidative stress can be altered by menopausal state. However, this study had too few female subjects to analyze the effects of menopausal state on the FORT value. Additional large-scale studies should be conducted to examine the relationship between oxidative stress and menopausal state in Korean women.
In this study, women, individuals who had high levels of hs-CRP, and metabolic syndrome patients exhibited higher oxidative stress. There have been few domestic studies on metabolic syndrome and oxidative stress in Korea. This study provides evidence that metabolic patients have higher oxidative stress than healthy individuals. Additional prospective intervention studies should be conducted to examine whether cured metabolic syndrome patients show reduction of the FORT values.
Notes
No potential conflict of interest relevant to this article was reported.