The Relationship between Metabolic Syndrome and Childhood Maternal Education Level, Job Status Findings from the Korean National Health and Nutrition Examination, 2007-2009
Article information
Abstract
Background
Childhood socioeconomic status (SES) is known to affect cardio-metabolic disease risk. However, the relationship between childhood SES and metabolic syndrome (MetS) remains uncertain. Therefore, we investigated the relationship between childhood SES, as measured by maternal education and occupational status and adult-onset MetS in the Korean population.
Methods
We examined the association between childhood SES, as measured by maternal education level and occupational status during an individual's childhood, and MetS in Korean adults aged 20 to 79 years who participated in the 2007-2009 Korean National Health Examination and Nutrition Survey. The components of MetS, including waist circumference, fasting glucose, lipid profiles, and blood pressure, were measured. Adjusted odds ratios (ORs) for MetS were calculated using multiple logistic regression models.
Results
Significant differences in the association between maternal education level, occupational status, and MetS were found between males and females. In females, the adjusted MetS OR for the highest maternal education quartile relative to the lowest quartile was 0.46 (0.21-0.99). Similarly, in females, the adjusted OR for individuals whose mothers worked when they were children relative to those whose mothers did not work was 1.23 (1.04-1.44). In males, no significant associations between maternal education, maternal occupational status, and MetS were found.
Conclusion
We found independent, positive associations between maternal education and occupational status and MetS in Korean females. These findings suggest that public health education targeting MetS prevention should be considered, especially among children with less opportunity for maternal support.
INTRODUCTION
Metabolic syndrome (MetS) is clinically defined as a clustering of metabolic indicators, including abdominal obesity, elevated blood pressure, glucose intolerance, and dyslipidemia.1) It is one of the most common risk factors for cardiovascular disease, cancer, and mortality.2,3) As rapid socioeconomic growth has led to lifestyle changes in Asian countries, including adoption of the Western diet and decreased physical activity, the prevalence of MetS has increased.2,4) Hormonal, genetic, and environmental factors are involved in MetS development, although the precise mechanisms responsible remain unknown.5) Because reducing the prevalence of MetS represents a major public health goal, the identification of modifiable risk factors, such as nutrition and exercise, is important.
Socioeconomic status (SES) is associated with metabolic disease risk. Recent studies have reported that low SES increases the risk of cardio-metabolic diseases, including diabetes mellitus (DM), cardiovascular disease, and MetS.6,7) Furthermore, significant associations between childhood and adult SES and cardio-metabolic pathologic conditions have been described.8,9,10,11) Although the precise mechanisms remain unknown, it is widely believed that the remodeling of neuro-endocrine and hormonal signaling pathways is involved.12,13) Because these pathways are key in the pathogenesis of MetS, we hypothesized that childhood SES may be related to adult-onset MetS. Previous studies investigating this association have been inconclusive. Several have reported that low parental education and occupational status increase MetS risk,14,15,16) but the opposite results have also been reported.17) In addition, culture significantly affects attitudes toward child-rearing.18) In Asia, maternal attitudes toward child-rearing display higher levels of affective-controlled characteristics and control than is observed in Western countries.18,19) To date, no large studies have evaluated the relationship between childhood SES, as assessed by maternal characteristics, and adult-onset MetS in an Asian population. Therefore, we evaluated the relationship between childhood SES, as measured by maternal education and occupational status, and MetS using data from the Korean National Health and Nutrition Examination Survey (KNHANES).
METHODS
1. Study Population
This study is a secondary analysis of data collected from the 2007-2009 KNHANES. KNHANES is a cross-sectional, nationally representative study performed by the Korean Ministry of Health and Welfare. The target population is non-institutionalized Korean civilians. The sampling unit was the household, and households were selected using a stratified, multistage probability sampling design. Participants were informed that their household had been randomly selected to participate in a survey performed by the Korean Ministry of Health and Welfare. They were given the right to refuse participation, in accordance with the National Health Enhancement Act supported by the National Statistics Law of Korea. All participants completed the health interview, health behavior survey, health examination, and nutrition survey, following a standardized protocol.
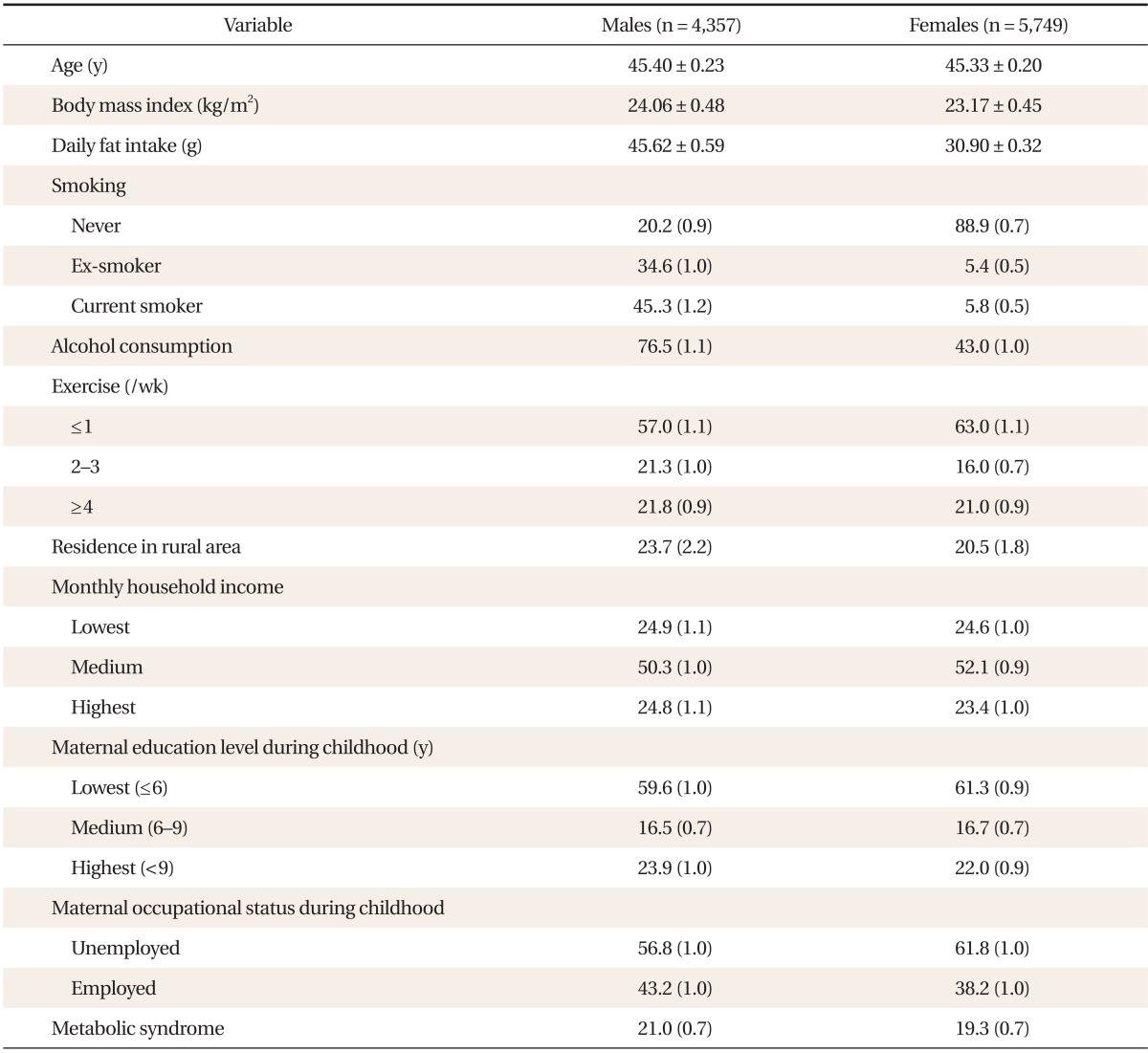
Of the subjects included in the 2007-2009 KNHANES, we excluded individuals younger than 20 and older than 79 years of age. We also excluded individuals without laboratory data, such as lipids and fasting plasma glucose, and those who had not fasted for at least 12 hours prior to blood sampling. Subjects with a history of cancer, cardiovascular disease, stroke, chronic liver disease, or chronic renal diseases were also excluded. After these exclusions, a total of 10,106 subjects (4,357 males, 5,749 females) were included in our final analysis.
2. Data Collection
Participants completed health examinations, including a questionnaire about medical history and health-related behaviors and anthropometric and biochemical measurements. All examinations were performed by trained medical staff who followed standardized procedures.
1) Survey on health-related behaviors and nutrition
Participants were asked about their lifestyle behaviors, including cigarette smoking, alcohol consumption, and dietary habits. Smoking status categories included: current smoker (someone who smokes cigarettes daily), ex-smoker (someone who smoked cigarettes in the past but does not currently smoke them), and never smoker (someone who has never smoked a cigarette). Alcohol consumption was defined as drinking alcohol more frequently than once per week or more than 70 g per week during the previous 1 year. KNHANES adopted the International Physical Activity Questionnaire to determine the frequency of physical activity. Physical activity was categorized according to exercise frequency: once per week or less, two or three times per week, and four times or more per week. Household income was used to assess current SES. Monthly household income was categorized into three groups: low (<918.30 USD), medium (918.30-3,213.90 USD) and high (≥3,214.00 USD). Dietary intake was assessed using 24-hour recall. Before testing, all subjects were instructed to maintain their usual dietary habits. Daily fat intake was calculated with Can-Pro 2.0, nutrient intake assessment software developed by the Korean Nutrition Society. If a subject was being treated for any disease, he or she was asked for the diagnosis and a list of medications being taken. Completed questionnaires were reviewed by trained staff and entered into a database.
2) Anthropometric measurements
Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with participants wearing light, indoor clothing without shoes. Body mass index was calculated as the ratio of weight (kg) to height (m2). Blood pressure was measured on the right arm using a standard mercury sphygmomanometer (Baumanometer; WA Baum, Copiague, NY, USA). Systolic and diastolic blood pressure (SBP and DBP) readings were recorded twice in a 5-minute interval and averaged.
3) Biochemical measurements
After a 12-hour overnight fast, blood samples were obtained from an ante-cubital vein. Fasting plasma glucose, triglycerides (TGs), and high density lipoprotein cholesterol (HDL-C) levels were measured using an ADVIA1650 Auto-analyzer (Siemens Medical Solutions Diagnostics, Erlangen, Germany). Participants provided written informed consent to allow the use of their blood samples in further analyses.
3. Assessment of Childhood Socioeconomic Status
Maternal education level and occupational status were used to assess childhood SES. The childhood SES interview was performed with a structured questionnaire on parental education and occupation. Childhood SES was defined as SES when the interviewee was 14 years old. Maternal education level was classified into three categories: elementary school (<6 years of schooling), middle-high school (range, 6 to 12 years), and university (more than 12 years). Years of education were determined based on the answer to this question: "What was the final education status of your mother?" Maternal occupational status was assessed based on the response to this question: "Did your mother have an occupation when you were 14 years old?" If the answer was yes, the mother was considered employed; if the answer was no, she was considered unemployed. Housewives were categorized as unemployed. Additionally, occupational status was categorized into manual versus non-manual occupations, using the Registrar General's classification.20) Professional, managerial, and semi-skilled non-manual occupations (e.g., legislators, senior officials, managers, professionals, technicians, associate professionals, clerks, service workers, shop workers, and market sales workers) were categorized as non-manual. Semi-skilled manual, partly skilled and unskilled occupations (e.g., agricultural and fishery workers, craft and related workers, plant and machine operators and assemblers, and elementary occupations) were categorized as manual.
4. Metabolic Syndrome Assessment
MetS and its components were diagnosed based on the National Cholesterol Education Program Adult Treatment Panel III Guidelines. We used ethnicity-specific values for waist circumference (WC), based on data from the World Health Organization and the Korean Society for the Study of Obesity.
MetS was defined as the presence of three or more of the following criteria: WC ≥ 90 cm in males or ≥ 80 cm in females; SBP ≥ 130 mm Hg, DBP ≥ 85 mm Hg, or taking an anti-hypertensive drug; fasting plasma glucose levels ≥ 100 mg/dL, taking any drug for hyperglycemia, or using insulin for low blood sugar; TG levels ≥ 150 mg/dL or taking any anti-dyslipidemic drug for hypertriglyceridemia; and HDL-C levels < 40 mg/dL in males or < 50 mg/dL in females or taking any anti-dyslipidemic drug for high HDL-C levels.
5. Statistical Analysis
Statistical estimates were weighted to represent the total population of Korea. We calculated standard errors using a method appropriate for the complex survey design and estimation procedure. Demographic and biochemical characteristics of the study population were analyzed using one-way analysis of variance or the chi-square test, stratified by sex. The association between childhood SES and MetS and its components was assessed first using the chi-square test. Data from the 2005 National Census from the Korea National Statistical Office were used to define the standard population.
Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) for MetS and its components were calculated using multivariate logistic regression models. To examine whether the associations between maternal education level, occupational status, and MetS were modified by other clinical variables, we tested for interactions on a multiplicative scale. Interaction terms between each clinical variable and maternal educational level or occupational status were included in the regression models. An interaction was considered statistically significant if the P-value was < 0.05.
All analyses were conducted using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, and a P-value of < 0.05 represented statistical significance.
RESULTS
1. Sample Characteristics
Clinical characteristics of the study subjects, stratified by sex, are shown in Table 1. The mean age was 45.40 ± 0.23 years for males and 45.33 ± 0.20 years for females. The prevalence of MetS was 21.0% ± 0.7% in males and 19.3% ± 0.7% in females.
2. Comparison of the Distribution of Childhood Maternal Educational Level and Occupational Status According to the Metabolic Syndrome by Gender
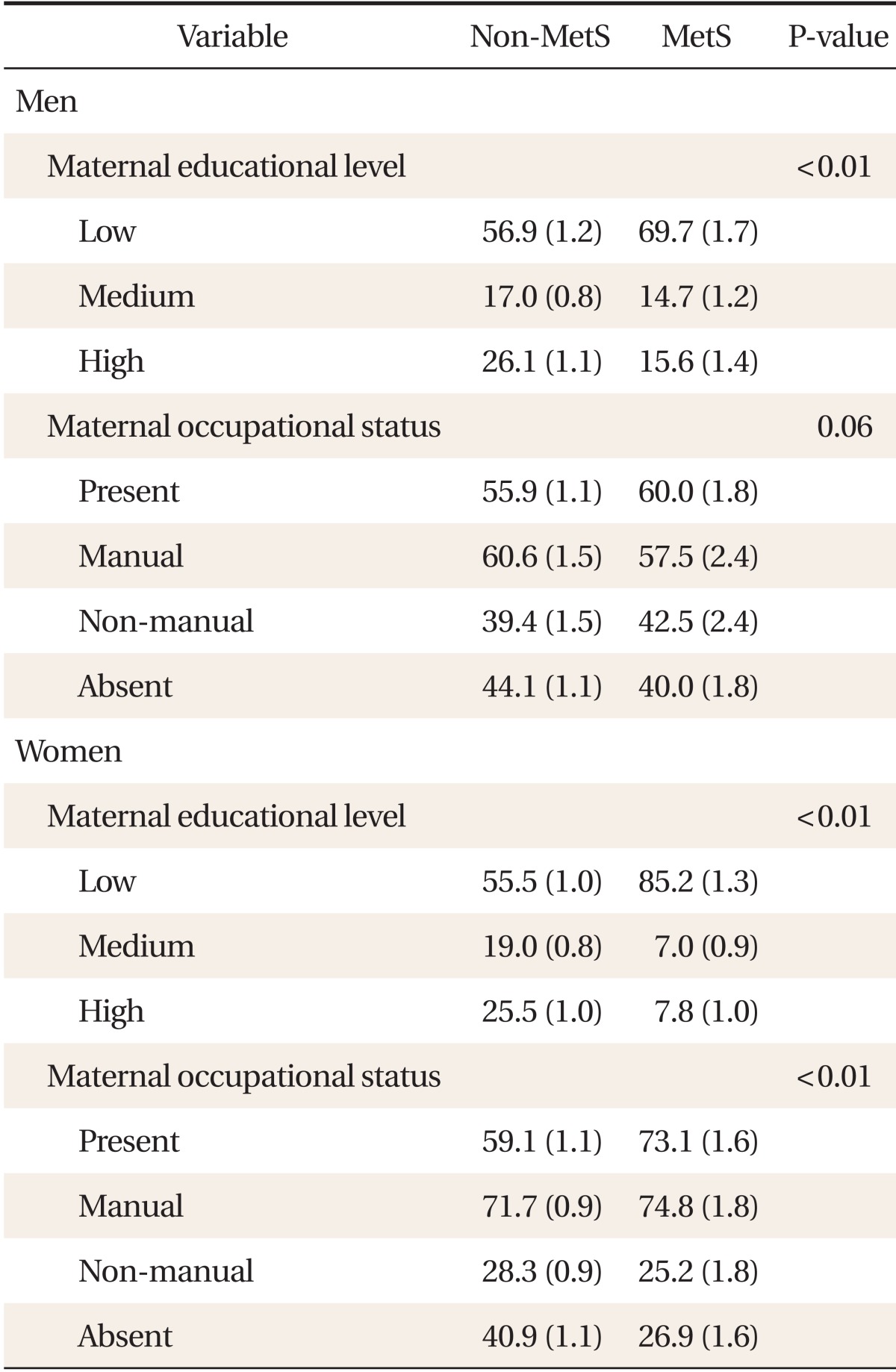
Table 2 presents the distribution of childhood maternal educational level and occupational status according to the MetS by gender. In males, the proportion of people with the highest childhood maternal education level was significantly higher in the non-MetS group. (P < 0.01). In females, the proportion of people with the highest childhood maternal education level was significantly higher in the non-MetS group (P < 0.01) and the maternal proportion of people with the presence of childhood maternal occupation was significantly higher in the MetS group (P < 0.01).
3. Association between Maternal Education Level and Occupational Status and Metabolic Syndrome
Tables 3 and 4 present ORs for developing MetS, according to maternal education level and occupational status. We found significant differences between males and females. After adjusting for potential confounders, we observed no significant associations between MetS and maternal education level or occupational status in males (Table 3). In females, however, a significant inverse association between maternal educational level and MetS was seen (Table 3). Relative to the lowest maternal education quartile, the adjusted MetS OR for the highest quartile was 0.46 (0.21-0.99). Relative to females whose mothers did not work during their childhood, the adjusted OR for those whose mothers did was 1.23 (1.04-1.44) (Table 4). The adjusted MetS OR for females whose mothers were employed in manual work relative to those whose mothers did not work was 1.34 (1.05-1.47). However, the odds of developing MetS were no higher in females whose mothers did non-manual work than those whose mothers did not work 1.16 (0.90-1.50).
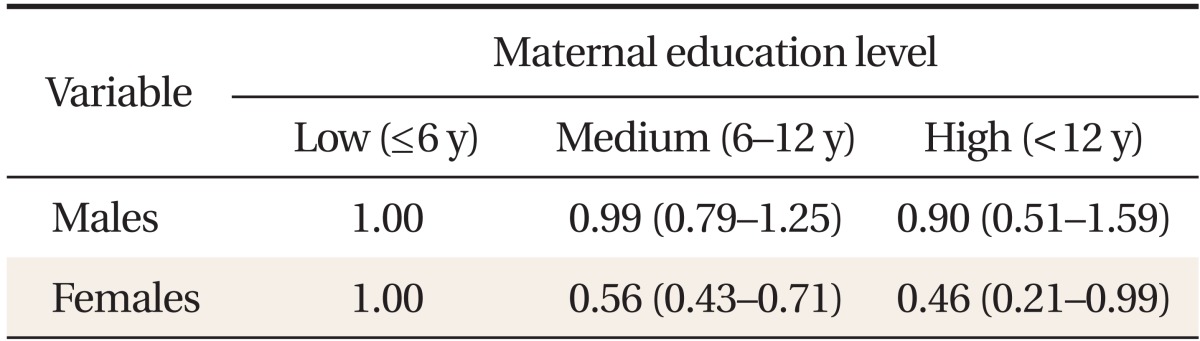
Odds ratios and 95% confidence intervals for metabolic syndrome, according to maternal education level*
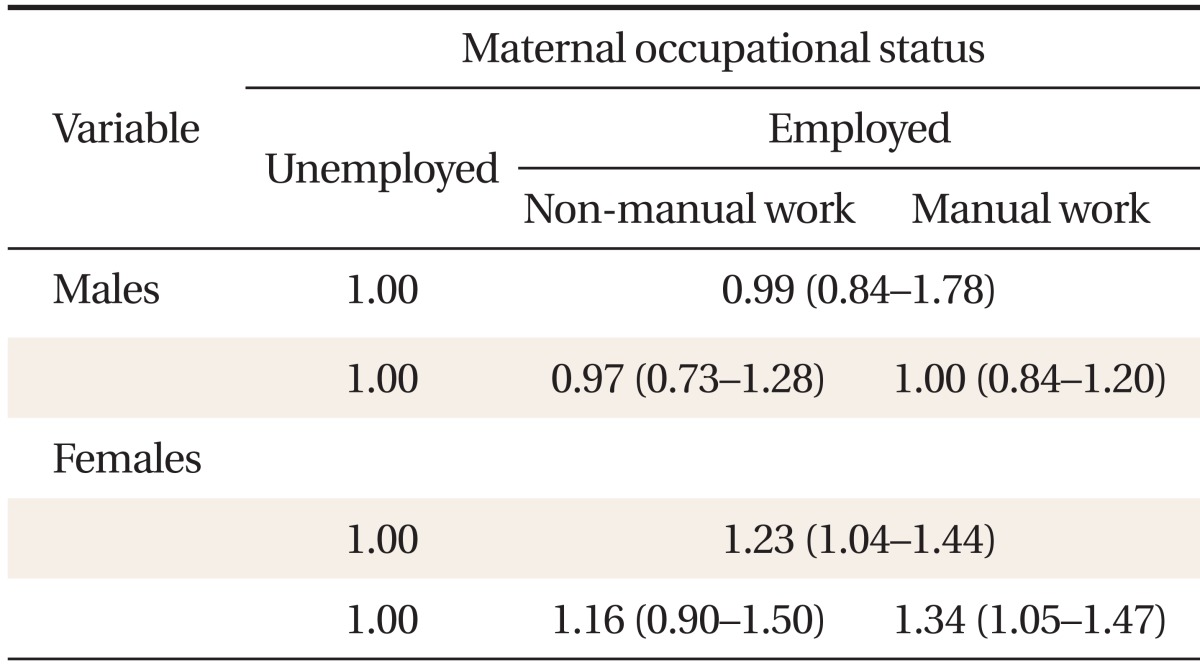
Odds ratios and 95% confidence intervals for metabolic syndrome, according to maternal occupational status*
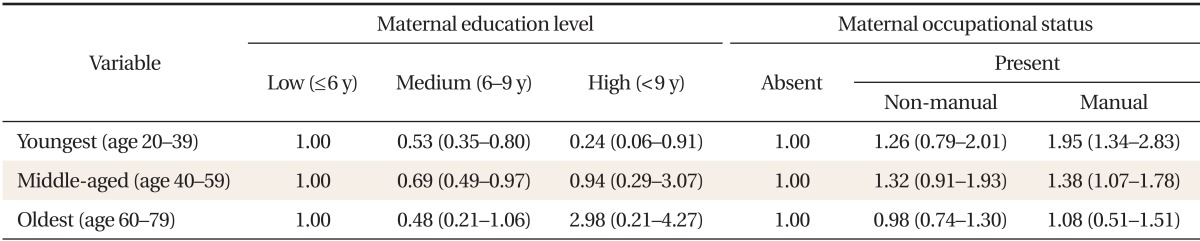
Using multiple logistic regression models for MetS, a significant statistical interaction between maternal SES and age was observed in females. Age modified the association between maternal education level, occupational status, and MetS (P < 0.05). Therefore, we stratified participants into three age cohorts: 20-39, 40-59, and 60-79 years. After stratification, no significant interaction between childhood SES and age were observed in females. Table 5 shows the adjusted MetS OR according to childhood SES for each female age cohort. In the youngest age cohort, the adjusted MetS OR for the highest vs. lowest maternal education levels was 0.24 (0.06-0.91). However, no significant associations were observed in the middle-aged and oldest age cohorts. In addition, in the young and middle-aged age cohorts, the adjusted odds of developing MetS for females whose mothers did manual work when they were children were significantly higher than for those whose mothers did not work: 1.95 (95% CI, 1.34 to 2.83) and 1.38 (95% CI, 1.07 to 1.78), respectively. However, such an association was not observed in the oldest age cohort. In males, no significant differences in the association between MetS and maternal education and occupational status were observed among the birth cohorts (data not shown).
DISCUSSION
We investigated the relationship between childhood SES and adult-onset MetS. In females, a significant inverse association between MetS and its components and maternal education level was identified. Females whose mothers worked during their childhood also had greater odds of developing MetS. These associations differed by birth cohort. The association between maternal SES and MetS was significant in the youngest birth cohort but not in older birth cohorts. In males, however, we detected no significant associations between MetS and maternal education level or occupational status.
Although childhood SES is thought to influence the development of adult-onset metabolic disturbance, recent studies have yielded conflicting results. A number of studies have found, as we did, that greater parental occupational status and education is associated with a decreased risk of adult-onset MetS,14,15,16) as well as DM and obesity.9,10,11) However, in the Pitt County cohort study, no significant association between childhood SES and adult-onset MetS was reported.17) Therefore, the relationship between childhood SES and MetS remains inconclusive, and there is also a lack of data from Asian countries. To the best of our knowledge, this is the first study to evaluate the relationship between the education level and occupational status of an individual's mother during their childhood and the odds of developing adult-onset MetS in a nationally-representative study of an Asian population.
The precise mechanisms by which childhood SES contributes to the emergence of MetS years later remain unclear. However, several possible mechanisms have been proposed. First, neuro-endocrine and inflammatory signaling systems may be involved. In addition, adults who grew up in low SES families display high cortisol secretion21,22) and chronic inflammation.23) The activation of pro-inflammatory signaling pathways and the inactivation of anti-inflammatory pathways may explain the chronic inflammatory status of these people. Because both high cortisol24) and inflammatory cytokine25) levels are known to play key roles in the pathogenesis of MetS, changes in neuroendocrine and inflammatory signaling systems during childhood may influence metabolic disturbances in later life. Additional experimental studies should be performed to identify the underlying mechanisms.
In addition, previous studies have reported that well-educated mothers have more knowledge about nutrition, physical activity, and other weight-related issues relevant to the development of adult obesity and MetS.26) Familial economic adversity is also known to be associated with poor quality parenting, higher levels of familial conflict, and childhood abuse, including harsh and neglectful parenting.27,28) Lehman et al.29) have reported that low familial SES influences adult metabolic function via hostility, poor social contact, and psychological depression. In our study, females whose mothers did not work during their childhood had lower odds of developing MetS than those who did, independent of maternal education level. It is unclear how to explain this relationship but nurturant parenting has educational, psychosocial, and bio-behavioral benefits for children facing adversity.30) A significant relationship between low maternal education status and adult-onset MetS has been reported previously; this association was weaker in people who recalled higher levels of nurturance from their mothers.31) Therefore, reduced opportunity to receive nurturant parenting may be the link between having a mother who worked and MetS.
In addition, we found a significant association between maternal SES and MetS in females. A previous study has found maternal attitude toward obesity control was not a significant predictor of male students' obesity control.32) The degree of self dissatisfaction with their weight had the most powerful effect in these students. By contrast, female students have been shown to be influenced by their mothers' health beliefs and attitudes toward obesity control.
This study had several limitations. First, it was cross-sectional. Thus, it is not possible to establish a causal relationship between maternal SES and MetS. Second, childhood SES data were obtained retrospectively, based on adult participants' recall. For this reason, it is possible that childhood SES was overestimated. Third, because this study was based upon questionnaire responses, we cannot fully exclude the effects of information bias. Finally, assessment of childhood SES was based on health interview responses about maternal education level and occupation. Because this information was based on participant recall, the possibility of information bias cannot be ruled out. Despite these limitations, we believe that our study is the first to assess the association between childhood SES and adult-onset MeS in the general population, using nationally representative data.
In conclusion, we have demonstrated a significant association between childhood SES, as measured by maternal education and occupational status and MetS in Korean females. Although we could not establish causality, our findings suggest that MetS prevention programs targeted to low-SES children and adolescents are needed.
Notes
No potential conflict of interest relevant to this article was reported.