|
 |
- Search
| Korean J Fam Med > Volume 35(2); 2014 > Article |
Abstract
Background
The glomerular filtration rate (GFR) decreases with age, while parathyroid hormone (PTH) increases. There are a few reports only on the relationship between GFR and PTH under the category of serum 25-hydroxyvitamin D (25[OH]D) concentration.
Methods
Using the Korea National Health and Nutrition Examination Survey (KNHANES) data, a cross-sectional study was conducted on the association between serum 25(OH)D concentration, GFR and PTH in Korean adults aged 50 years or older. Serum PTH concentration was compared to the tertiles of GFR after adjustment for relevant variables. In addition, the serum PTH concentration was compared with the GFR under the category of serum 25(OH) D concentration (<20, 20-30, >30 ng/mL).
Results
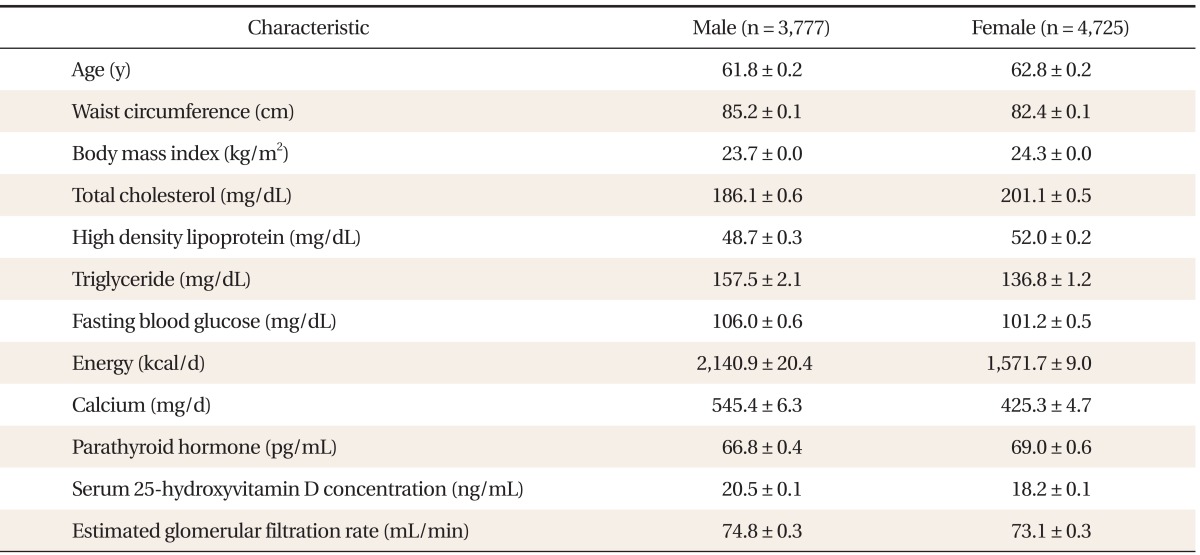
The mean estimated GFR (eGFR) was 74.8 mL/min in men and 73.1 mL/min in women. The mean PTH and 25(OH) D was 66.8 pg/mL, 20.5 ng/mL in men and 69.0 pg/mL, 18.2 ng/mL in women. The serum PTH concentration showed a significant negative correlation with the serum 25(OH) D and eGFR in both genders. The serum PTH concentration significantly increased at the lower tertile of eGFR in male adults In addition, a decrease of serum PTH concentration was marked in the vitamin D sufficient male adults (>30 ng/mL).
Vitamin D is a fat-soluble vitamin with various health benefits.1,2,3) The traditional function of vitamin D is to increase the intestinal absorption of calcium for proper mineralization of bone,4) to regulate blood pressure by various mechanisms5,6,7,8,9) and to stimulate kidney reabsorption of calcium and phosphate, mainly through its dominant active metabolite 1,25-dihydroxyvitamin D.10)
Vitamin D deficiency has been recognized as a pandemic with a myriad of health consequences.11,12) Low vitamin D status has been associated with an increased risk of type 1 diabetes mellitus, cardiovascular diseases, certain cancers, cognitive declines, depressions, pregnancy complications, autoimmunity, allergies and even frailty.13,14) Parathyroid hormone (PTH) is an important hormone for bone health which maintains the normal serum concentrations of calcium and phosphate. Persistently increased PTH concentrations will increase bone turnover, causing negative bone balance and an increased fracture risk.15) This leads not only to bone loss but to increased bone remodeling also, which is recognized as an important contributor to bone strength.16) PTH is regulated through levels of vitamin D and calcium and vitamin D insufficiencies are generally associated with an increase in PTH.17) Although the most-studied and best-known function of vitamin D together with PTH, is related to bone metabolism,18) many studies show evidence of the relationship between low levels of 25-hydroxyvitamin D (25[OH]D) and chronic kidney disease (CKD).19,20,21) PTH is negatively correlated with glomerular filtration rate (GFR).22,23) Elevated PTH levels, considered one of the earliest markers of abnormal bone mineral metabolism in CKD, have been frequently reported in clinic-based studies in patients with renal insufficiency.24,25,26)
Actually, there is little known about the relationship between serum 25(OH)D concentration, serum PTH concentration and estimated GFR (eGFR) in Korean adults. Therefore, the aim of this study was to examine the relationship between serum 25(OH)D concentration, serum PTH concentration and eGFR in a large sample of Korean adults aged over 50 using the Korea National Health and Nutrition Examination Survey (KNHANES) which was conducted between 2009 and 2011.
KNHANES, conducted periodically by the Korea Centers for Disease Control and Prevention since 1998, provide comprehensive information about the health status, health behavior, nutritional status and sociodemographic data of 600 national districts in Korea. For the present study data were used from the fourth (IV-3, 2009), fifth (V-1, 2010) and fifth (V-2, 2011) KNHANES consisting of a health interview survey, a nutrition survey and a health examination survey. Data about demographic characteristics, diet and health related variables were collected through personal interviews and self-administered questionnaires. Physical examinations, blood samplings and urine samplings were carried out at a mobile examination center. Data samplings containing serum 25(OH)D concentrations, serum PTH concentrations and serum creatinine (Cr) were used in this cross-sectional analysis. The eGFR (mL/min) was calculated from age, sex, body weight, and serum Cr concentration using the Cockcroft-Gault formula. From an initial total of 37,753 men and women, 13,682 subjects (5,872 men and 7,810 women) were evaluated aged 50 years and older with serum 25(OH)D, serum PTH and eGFR. From this, 4,551 subjects were excluded because of missing data on serum 25(OH)D (1,684 subjects), serum PTH (4,532 subjects), serum Cr (1,371 subjects) or a missing body weight (572 subjects). For 3,608 subjects combined missing data were noted. Additionally, 598 subjects were also excluded because of the use of vitamin or mineral supplements. Among them, 20 subjects were excluded because of underlying chronic renal failure and too-high serum PTH concentrations (>300 pg/mL). Finally the analysis was conducted with the data of 8,502 subjects (3,777 males and 4,725 females) as shown in Figure 1. All participants provided written informed consent before the survey.
Blood samples were collected from the antecubital vein of each participant after fasting overnight. The blood samples were properly processed, refrigerated at 2Ōäā to 8Ōäā and shipped to the Central Testing Institute (NeoDin Medical Institute, Seoul, Korea). Serum 25(OH)D concentration was measured with a radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA) using a g-counter (1470Wizard; PerkinElmer, Turku, Finland). Serum 25(OH) D was measured in the same institute. Quality control was conducted every other week throughout the analysis period to minimize analytical variation. Serum 25(OH) D was divided into three groups: a group with vitamin D deficiency (<20 ng/mL), a group with vitamin D insufficiency (<20-30 ng/mL) and a group with vitamin D sufficiency (>30 ng/mL) according to the American Guidelines.27)
Intact PTH (iPTH) was measured using the N-tact PTH assay with LIAISON (Diasorin) by chemiluminescence immunoassay method. An elevated serum iPTH was defined as Ōēź70 pg/mL based on the cut-point for the definition of an elevated iPTH according to the K/DOQI guidelines. The eGFR was estimated by the equation of [(140 - age) ├Ś (body weight)] / (serum Cr ├Ś 72) with a factor of 0.85 applied for women. Analyses of fasting glucose, total cholesterol, high density lipoprotein and triglycerides were performed by Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) and by enzymatic methods using commercially available kits (Daiichi, Tokyo, Japan). Nutrient intake, including the total intake of calories and calcium, were assessed with a 24-hour dietary recall questionnaire administered by a trained dietician. The results were calculated using the Food Composition Table developed by the National Rural Resources Development Institute (7th revision).28) Contents of dietary supplements were not documented in the KNHANES.
Physical examinations were performed by a trained examiner following a standardized procedure. Body weight and height were measured in light indoor clothing without shoes to the nearest 0.1 kg and 0.1 cm, respectively. Physical activity was assessed by a questionnaire and categorized as "yes" or "no" with "yes" meaning 30 minutes of moderate physical activity three or more times in the last week in which the subject was tired compared to ordinary levels. Menarche age was determined by a health questionnaire administered by a trained examiner. Women were classified into women in menopausal status and women with a hormone replacement therapy.
Complex sample analysis after data weighting was used for the KNHANES data for the assessment of all values following the statistics guidance of the Korea Centers for Disease Control and Prevention. After the assessment, the general characteristics for both genders were presented including age, waist circumference, body mass index, total cholesterol, high density lipoprotein, triglycerides, fasting blood glucose, daily total energy intake, daily calcium intake, serum parathyroid hormone concentrations, serum 25(OH)D concentrations and eGFR. To evaluate the association between serum 25(OH)D concentrations and serum PTH concentration with eGFR a partial correlation analysis was performed in female and male subjects after adjustment for age, body mass index (BMI), job, alcohol intake, smoking status and moderate physical activity. Serum PTH did not have normal distribution, however, we did not make a log-transformation, because log-transformed PTH did not show normal distribution either and authors decided that using PTH values as they were had more clinical relevance. We compared the difference in serum PTH concentrations by eGFR according to the serum 25(OH) D concentration category (<20, 20-30, and <30 ng/mL) in both genders. In addition, we evaluated the interaction of serum PTH and eGFR according to the serum 25(OH)D concentration category in both genders. Finally, to determine the association between eGFR and serum PTH concentration, eGFR was divided into tertiles in each gender. In these eGRF categories, the differences in serum PTH concentrations were determined across the tertiles with the analysis of covariance test after adjustment for age, BMI, job, alcohol intake, smoking, moderate physical activity, daily total energy intake, daily calcium intake, serum 25(OH) D concentration and additionally for women, menopausal status, the use of oral contraceptive, or a hormone replacement therapy. All P-values were P for trend used for assessment of the significance of all analysis and P < 0.05 was considered significant. Data were analyzed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
A total of 8,502 people were enrolled in this trial. The general characteristics of the study participants are shown in Table 1. The serum 25(OH)D concentration and the eGFR in male adults were on average higher than in females (20.5 ┬▒ 0.1 ng/mL vs. 18.2 ┬▒ 0.1 ng/mL and 74.8 ┬▒ 0.3 mL/min vs. 73.1 ┬▒ 0.3 mL/min, respectively). The Serum PTH concentration was higher in female (69.0 ┬▒ 0.6 pg/mL) than in male adults (66.8 ┬▒ 0.4 pg/mL).
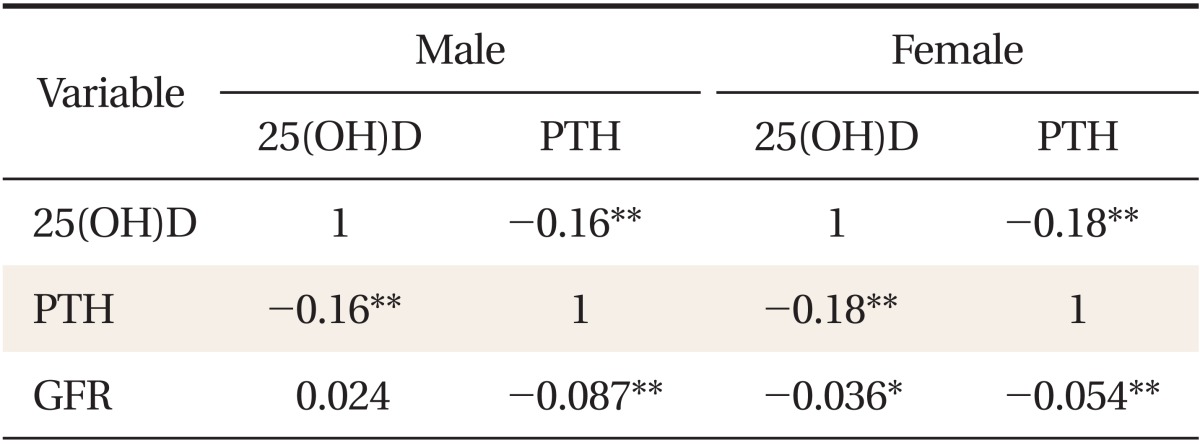
To determine the association between serum 25(OH)D concentration and serum PTH concentration with eGFR, a partial correlation analysis was conducted (Table 2). A significant relationship was shown in both genders between serum 25(OH) D and the PTH concentration (r = -0.16, P < 0.001 in male adults; r = -0.18, P < 0.001 in female adults). Additionally to an inverse relationship between serum 25(OH)D and serum PTH, serum PTH showed a significantly inverse correlation with eGFR in both genders (r = -0.087, P < 0.001 in male; r = -0.054, P < 0.001 in female adults).
Serum PTH concentration in men significantly increased with the reduction of eGFR (P for trend = 0.001). Whereas, serum PTH concentration in the lowest eGFR group in female adults increased without statistical significance. Further, the effect of serum 25(OH)D concentration on the relationship between eGFR and serum PTH was compared with serum 25(OH) D (<20, 20-30, >30 ng/mL). Even though eGFR decreased, serum PTH concentration remained low in both genders, except in the top category of 25(OH)D in female adults (Figure 2A, B). However, in the evaluation of interaction between PTH and GFR, it was shown the significant in the serum 25(OH) D <20 (P = 0.003), 20-30 (P = 0.129), >30 (P = 0.571) ng/mL in male adults and 25(OH)D <20 (P < 0.001), 20-30 (P = 0.005), >30 (P = 0.006) ng/mL in female adults, respectively (data not showing). To explore the association between eGFR and serum PTH concentration, eGFR was divided into tertiles in both genders. Data were adjusted for age, BMI, job, alcohol intake, smoking status, moderate physical activity, daily total energy intake, daily dietary calcium intake, and serum 25(OH)D concentration. The concentration of serum PTH concentration in each eGFR tertile is shown in Table 3.
This study examined serum concentrations of 25(OH) D and serum PTH in relation to eGFR in subjects aged over 50 using KNHANES data assessed between 2009 and 2011. This cross-sectional study reveals that low levels of serum 25(OH)D are common in the study population. As expected, an inverse relationship between serum 25(OH)D and serum PTH was found also. This reflects the fact that PTH secretion is stimulated by decreased vitamin D levels. The serum PTH showed a significant but weak negative correlation with eGFR in both genders additionally. The subjects of the lowest eGFR group presented significantly higher serum PTH concentration compared with those of the middle and highest male adult groups. On the other hand, the serum PTH concentration increased in the lowest eGFR group more than in the middle and highest female adult groups without statistical significance. In this study, the majority of female participants (94.2%) were categorized into the vitamin D insufficiency or deficiency groups. Therefore, the results may be affected due to a skewed distribution of vitamin D levels and a lack of adequate numbers of sufficient vitamin D levels. Furthermore, eGFR reductions amplified the serum PTH response for a given 25(OH)D level, especially for the low of 25(OH)D levels compared to those with middle and highest serum 25(OH)D levels in both groups.
The elderly population is at particular at risk for clinical complications related to low 25(OH)D levels. Because of changes in lifestyle, such as types of clothing worn and less outdoor activity, sun exposure is usually limited with increasing age. Diet may also become less varied and with a lower natural vitamin D content. More importantly, during aging, the production of vitamin D after exposure to solar UV-B radiation decreases because of atrophic skin changes with a reduced amount of its precursor 7-DHC.29,30) Also, high latitude (37 degrees north) and cultural preferences may contribute to low serum 25(OH)D levels.
There are emerging data associating high levels of serum PTH with morbidity and increased mortality. Raised serum PTH has been associated with weight gain and adiposity31,32) and with poor muscle function.33) A high serum PTH was also associated with increased mortality34) and time to first fall35) in a frail elderly population. Although the evidence was far less than for vitamin D, a community-based prospective study predicted an elevated PTH level for cardiovascular mortality. By which mechanism the serum PTH may influence these variables is unknown. A graded association between lower eGFR and a higher prevalence of serum PTH > 65 pg/mL was reported among a moderate-size population of patients (n = 1,814) recruited from clinical offices in the United States and Canada and enrolled in the Study for the Early Evaluation of Kidney Disease.32) The current study extends previous findings to a large, nationally representative sample of the Korean population. In this study, the average serum PTH concentration was more than Ōēź70 pg/mL (71.4 ┬▒ 1.8 pg/mL in man and 71.3 ┬▒ 1.2 pg/mL in woman) in the lowest eGFR group (17.4-66.1 mL/min). Lowest eGFR group included stage 3 chronic kidney disease (eGFR >30-59 mL/min/1.73 m2), stage 4 chronic kidney disease (eGFR >15-29 mL/min/1.73 m2) and stage 5 chronic kidney disease (eGFR <15 mL/min/1.73 m2) according to the Kidney Disease Outcome Quality Initiative (K/DOQI) staging,36) patients did not necessarily have chronic kidney diseases although. These results demonstrate a strong association between moderate to severe CKD and a higher prevalence of elevated serum PTH levels in Korean adults aged 50 and above. With the findings of the present study a mild elevation of PTH levels could not be interpreted as a risk factor for deterioration in renal function.
A deficiency of 25(OH)D in the low eGRF group may aggravate elevations in the serum PTH and the prevention of vitamin D deficiency may reduce the frequency and severity of secondary hyperparathyroidism. The majority of the adults with a reduced eGFR had 25(OH)D levels <30 ng/mL in this study. Even moderate reductions in renal function have been associated with significant increases in the risk of hip fractures.37,38) An assessment of the serum 25(OH)D status and a correction of 25(OH)D insufficiency are recommended among patients with elevated PTH above the target range. A serum 25(OH)D level with at least 20 ng/mL is required to normalize PTH levels, to minimize the risk of osteomalacia and for optimal bone and muscle function. Many experts consider 30 ng/mL as the threshold for optimal bone health.39) There is potentially a great upside (in terms of overall health improvement and well-being) to increasing the serum 25(OH)D levels above 30 ng/mL.40) The majority of the current study population is likely to benefit from a higher vitamin D intake considering that a vitamin D dosage of 800 IU/d resulted in increases of serum 25(OH)D levels to greater than 30 ng/mL in 97.5% of postmenopausal women as shown in a recent study.41) The current adequate intake (AI) for vitamin D in Korea is 400 IU/d (50 years old).42) An increase of vitamin D intake to levels above the current Korean AI is needed for the normalization of PTH levels, optimal bone mass and an improvement of overall health and well-being.
This study has several limitations. Subjects were limited to adults over 50 years. Therefore, the result of the study may be not applicable to younger adults. Most study subjects were in a state of vitamin D deficiency or insufficiency with their vitamin concentration levels in serum and the narrow range of vitamin D concentrations might affect the result. The season of vitamin D measurement was not adjusted to the study. The prevalence of vitamin D deficiency and insufficiency is affected by seasonal variations and latitudes. It increases in the late winter as well as in the spring and decreases in the summer.43) However, these factors may not be the confounders of the association because all participants in KNHANES were randomly selected through all seasons. Data for 1,25(OH)2D were also not collected to further explore the influence of this important metabolite on PTH levels. It is true that serum 25(OH)D is inactive and it may not represent the actual total body store of the main regulatory hormone 25(OH)D or 1,25(OH)2D.44) However, 25(OH)D is the substrate for 1,25(OH)2D, presenting in circulation with a about 1,000 times higher concentration than 1,25(OH)2D32) and 1,25(OH)D. It is not routinely measured in clinical practice and it will not necessarily limit our observations about the relationship between 25(OH)D and PTH. Finally, we were not sure why serum PTH decrease was significant according to the category of serum 25(OH)D concentration and eGFR only in male adults. The difference of body composition, calcium intake, and other metabolic parameters might be some of the causes of gender difference in serum PTH concentration.
In conclusion, this study demonstrated that serum PTH concentration showed negative correlation with eGFR; however, serum PTH increase may be minimized by maintaining proper serum 25(OH)D concentration under similar eGFR status in Korean adults aged 50 and above. Therefore, maintaining serum 25(OH)D concentration to avoid a vitamin D deficiency is very important to keep serum PTH at lower concentrations.
ACKNOWLEDGMENTS
We thank all the members of the Korea Institute for Health and Social Affairs who conducted the national survey by the Division of Health and Nutrition Survey, Korea Centers for Disease Control & Prevention for the support of the KNHANES data analysis.
References
3. Rosen CJ. Clinical practice: vitamin D insufficiency. N Engl J Med 2011;364:248-254. PMID: 21247315.


4. Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep 2008;10:110-117. PMID: 18460265.



5. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 2004;89-90:387-392. PMID: 15225806.


6. Vaidya A, Forman JP. Vitamin D and hypertension: current evidence and future directions. Hypertension 2010;56:774-779. PMID: 20937970.


7. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011;57:63-69. PMID: 21115878.


8. Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J 2012;33:2980-2990. PMID: 22267242.


9. Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009;6:621-630. PMID: 19687790.


10. Metzger M, Houillier P, Gauci C, Haymann JP, Flamant M, Thervet E, et al. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: quest for a threshold. J Clin Endocrinol Metab 2013;98:2922-2928. PMID: 23633202.


11. Holick MF. Vitamin D: extraskeletal health. Rheum Dis Clin North Am 2012;38:141-160. PMID: 22525849.


12. Hossein-nezhad A, Holick MF. Optimize dietary intake of vitamin D: an epigenetic perspective. Curr Opin Clin Nutr Metab Care 2012;15:567-579. PMID: 23075936.


13. Smit E, Crespo CJ, Michael Y, Ramirez-Marrero FA, Brodowicz GR, Bartlett S, et al. The effect of vitamin D and frailty on mortality among non-institutionalized US older adults. Eur J Clin Nutr 2012;66:1024-1028. PMID: 22692022.


14. Holick MF. Nutrition: D-iabetes and D-eath D-efying vitamin D. Nat Rev Endocrinol 2012;8:388-390. PMID: 22640996.


15. Favus MJ. Postmenopausal osteoporosis and the detection of so-called secondary causes of low bone density. J Clin Endocrinol Metab 2005;90:3800-3801. PMID: 15917489.


17. Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005;294:2336-2341. PMID: 16278362.


19. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 2005;289:F8-F28. PMID: 15951480.


20. Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial 2005;18:266-275. PMID: 16076348.


21. Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int 2006;70:654-659. PMID: 16807549.


22. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 Suppl 3):S1-S201. PMID: 14520607.

23. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-1930. PMID: 21646368.


24. Torres A, Lorenzo V, Hernandez D, Rodriguez JC, Concepcion MT, Rodriguez AP, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int 1995;47:1434-1442. PMID: 7637272.


25. Fajtova VT, Sayegh MH, Hickey N, Aliabadi P, Lazarus JM, LeBoff MS. Intact parathyroid hormone levels in renal insufficiency. Calcif Tissue Int 1995;57:329-335. PMID: 8564794.


26. Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int 1999;56:1084-1093. PMID: 10469378.


27. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713-716. PMID: 15776217.


28. National Rural Resources Development Institute. Rural development administration: food composition table. 7th ed. Suwon: National Rural Resources Development Institute; 2006.
29. Godar DE, Pope SJ, Grant WB, Holick MF. Solar UV doses of young Americans and vitamin D3 production. Environ Health Perspect 2012;120:139-143. PMID: 21852226.


30. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest 1985;76:1536-1538. PMID: 2997282.



31. Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr 2002;21:146S-151S. PMID: 11999543.


32. McCarty MF, Thomas CA. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med Hypotheses 2003;61:535-542. PMID: 14592784.


33. Garber AJ. Effects of parathyroid hormone on skeletal muscle protein and amino acid metabolism in the rat. J Clin Invest 1983;71:1806-1821. PMID: 6306055.



34. Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab 2004;89:5477-5481. PMID: 15531500.


35. Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab 2004;89:1572-1576. PMID: 15070914.


36. Pepe J, Romagnoli E, Nofroni I, Pacitti MT, De Geronimo S, Letizia C, et al. Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos Int 2005;16:805-812. PMID: 15551058.


37. Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: the Parathyroid Epidemiology and Audit Research Study (PEARS). Clin Endocrinol (Oxf) 2011;75:169-176. PMID: 21158894.


38. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006;17:3223-3232. PMID: 17005938.


39. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18-28. PMID: 16825677.


40. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc 2013;88:720-755. PMID: 23790560.



41. Gallagher JC, Sai A, Templin T 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012;156:425-437. PMID: 22431675.


42. The Korean Nutrition Society. Dietary reference intakes for Koreans 2010. Seoul: The Korean Nutrition Society; 2010.
43. Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 2011;12:4-18. PMID: 20795941.


44. Chausmer AB. Dose response to vitamin D supplementation in postmenopausal women. Ann Intern Med 2012;157:384PMID: 22944879.


Figure┬Ā1
Flow diagram of subject inclusion and exclusion in this study. KNHANES: Korean National Health and Nutrition Examination Survey, PTH: parathyroid hormone.
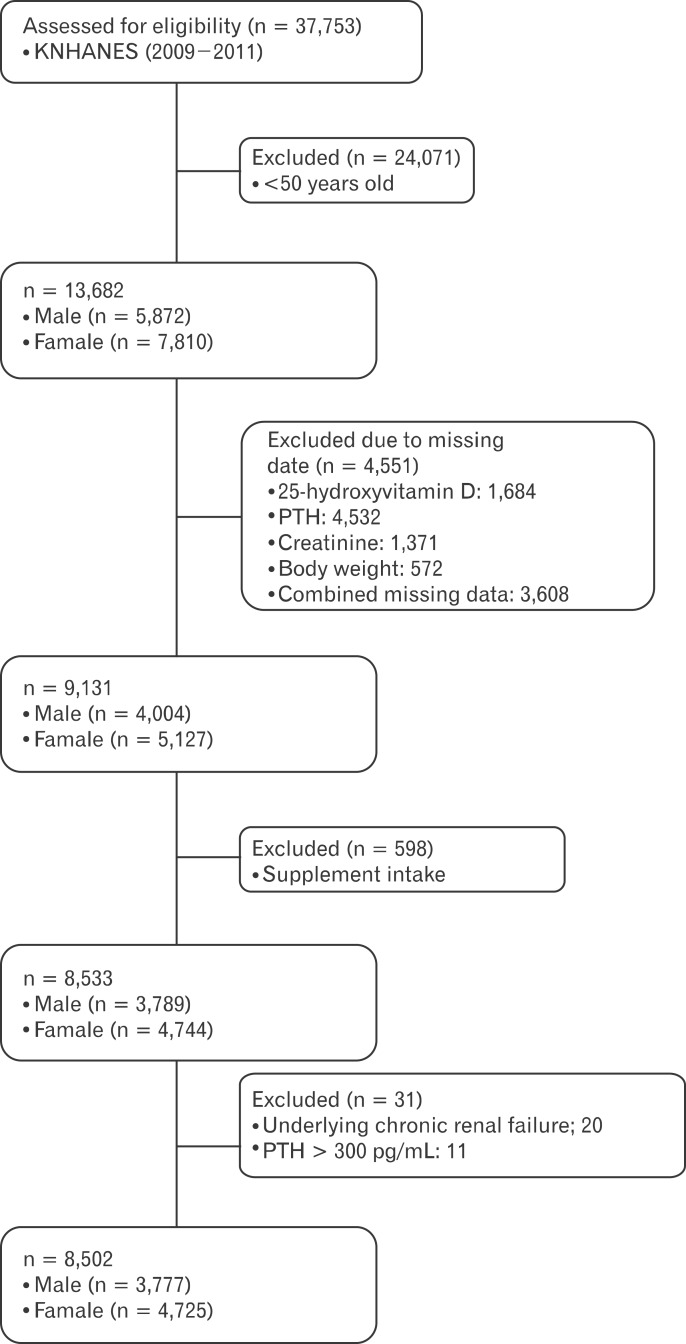
Figure┬Ā2
Comparison of serum PTH concentration by GFR according to serum 25(OH)D category in Men (A, B, C), and Women (D, E, F) adults. A (men) and B (women) show the difference of serum PTH by eGFR according to the serum 25(OH)D category (<20 ng/mL, 20-30 ng/mL, <30 ng/mL). This scatter-plot curves show that serum PTH concentration increases as the eGFR decreases at the serum 25(OH) D <20 ng/mL in both genders. However, as the serum 25(OH)D increases, serum PTH concentration decreases, even though the eGFR decreases. This figures were made by no relevant variables adjustment, but simple relation between serum PTH and eGFR according to the serum 25(OH)D category. The interaction between PTH and eGFR, however, was significant in the serum 25(OH)D <20 (P = 0.003), 20-30 (P = 0.129), >30 (P = 0.571) ng/mL in male adults and 25(OH)D <20 (P < 0.001), 20-30 (P = 0.005), >30 (P = 0.006) ng/mL in female adults, respectively. 25(OH)D: 25-hydroxyvitamin D, PTH: parathyroid hormone, GFR: glomerular filtration rate, eGFR: estimated GFR.
Table┬Ā3
Serum PTH concentration by GFR tertiles
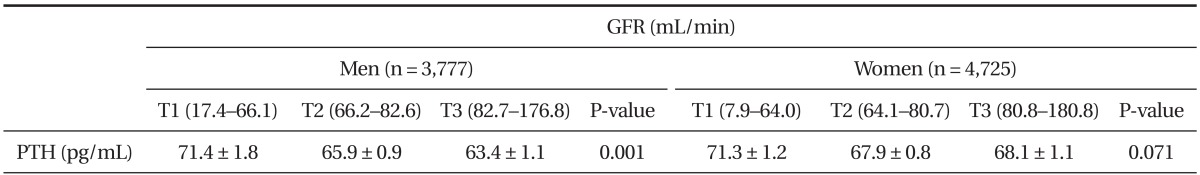
Values are presented as mean ┬▒ standard error of serum PTH concentration. P-values are P for trend from analysis of covariance test after adjustment with age, body mass index, job, alcohol intake, smoking status, moderate physical activity, daily total energy intake, daily dietary calcium intake and serum serum 25-hydroxyvitamin D concentration and menopause, hormone replace therapy, oral contraceptive intake in case of women.
PTH: parathyroid hormone, GFR: estimated glomerular filtration rate.
- TOOLS