Association between Smoking and Periodontal Disease in Korean Adults: The Fifth Korea National Health and Nutrition Examination Survey (2010 and 2012)
Article information
Abstract
Background
This study aimed to evaluate an association between smoking, smoking cessation, and periodontal disease in Korean adults.
Methods
The data were collected from 8,336 participants, aged between 20 and 64 years, who participated in the fifth Korea National Health and Nutrition Examination (2010 and 2012). Smoking status was assessed using self-administered questionnaires. Periodontal disease was defined as a community periodontal index ≥3 points. Logistic regression analysis was used to evaluate an association between smoking, smoking cessation, and periodontal disease after adjusting for age, sex, education, monthly income, diabetes, obesity, alcohol intake, and frequency of tooth brushing.
Results
The risk of periodontal disease was higher among current smokers (odds ratio [OR], 1.49; 95% confidence interval [CI], 1.21-1.83) than never smokers. Among current smokers, the risk of periodontal disease was increased in smokers of ≥10 cigarettes/d, ≥20 years duration, and >10 pack-years compared with never smokers (P<0.05). Among former smokers, the risk of periodontal disease after 10 years since cessation declined to 0.56 (95% CI, 0.42-0.75) compared with current smokers and was indistinguishable statistically from never smokers.
Conclusion
Periodontal disease is significantly associated with smoking status in Korean adults.
INTRODUCTION
Periodontal disease leads to a breakdown of the connective tissue and bone that anchor the teeth1) and can eventually lead to the loss of teeth.2) It is estimated that 1 in 2 men and 1 in 3 women over the age of 50 years in Korea have periodontal disease.3)
Periodontal disease has been associated with systemic disease and increases the risk of diabetes, preterm low birth weight delivery, cardiovascular disease, osteoporosis, and pneumonia. It has recently been reported that periodontal disease is also correlated with rheumatoid arthritis.45) Periodontal inflammation was also reported to have a negative effect on glycemic control in diabetic patients56) and is a risk factor not only for nosocomial pneumonia7) but also for communityacquired pneumonia.8) Periodontal tissues may serve as a reservoir of bacteria, bacterial products, and inflammatory and immune mediators, which can interact through blood vessels with other organ systems remote from the oral cavity.9)
Known risk factors for periodontal disease include male sex, older age, low income-level, low education level, diabetes, obesity, smoking, alcohol drinking, stress, and low serum 25-hydroxyvitamin D3 concentrations. 101112)
Korean studies have studied only smoking as a potential confounder of periodontal disease or simply analyzed the association between periodontal disease and smoking status (current smokers, former smokers, and never smokers).1213) However, there has been a lack of studies on the dose-response relationship between smoking and periodontal disease or the effects of smoking cessation.
The present study aimed to investigate the association and dose-response relationship between smoking and periodontal disease, as well as the association between smoking cessation and periodontal disease, in Korean adults.
METHODS
1. Study Population
The subjects of the present study were selected from among 9,204 adults aged 20–64 years old who participated in the first and the third year (2010 and 2012) of the fifth Korea National Health and Nutrition Examination Survey (KNHANES).14) Of these, 868 were excluded owing to missing data regarding periodontal disease, age, sex, household income quartile, education level, diabetes status, obesity status, lifetime and current alcohol drinking status, lifetime and current smoking status, and frequency of tooth brushing. The remaining 8,336 individuals were enrolled in the study. Analysis of long-term smoking for current smokers was conducted for 1,877 adults, excluding 5 who had missing data for the age of smoking initiation. Cumulative smoking exposure of current smokers was analyzed for 1,875 adults, excluding 7 who had missing data for the age of smoking initiation, average daily smoking amount for current smokers, and number of smoking days in the prior month for intermittent smokers. Analysis of smoking cessation for former smokers was performed for 1,254 adults, excluding 9 who had missing data for the age of smoking initiation and smoking duration among former smokers. The second year (2011) of the fifth KNHANES was excluded because the raw data of periodontal examinations were not disclosed.
2. Study Variables
A household health survey of age, sex, number of household members, and household income was conducted by face-to-face interviews, in which one member answered on behalf of the entire household. Surveys on education and comorbidities such as diabetes were conducted by a direct interview with the individual. A self-administered survey was used for the health behavior questionnaire that included questions on smoking and alcohol drinking; this method was intended to exclude the possibility of under- or overestimation due to the potentially sensitive nature of the questions.15) The education level was assessed by the highest diploma. Income level was classified by quartiles based on monthly average family equivalent income (total household income divided by the square root of the number of household members). For the survey of diabetes status, subjects were asked whether they had ever been diagnosed with diabetes. Obesity status was classified by body mass index (BMI) into underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), or obese (BMI ≥25 kg/m2).16) Alcohol drinking was classified by whether or not alcohol was consumed at least once a month during the prior year. The frequency of tooth brushing on the day before the survey was categorized as 0–1, 2, or ≥3 times.
Subjects who reported smoking at least 100 cigarettes in their lifetime and who smoked either every day or on some days at the time of the survey were defined as current smokers. Those who reported smoking at least 100 cigarettes in their lifetime and who did not smoke at all at the time of the survey were defined as former smokers. Those who had never smoked or had smoked less than 100 cigarettes in their lifetime were defined as never smokers.17) Average daily cigarette consumption of current smokers was determined according to the answers to the following question "How many cigarettes did you smoke on average per day?" For intermittent smokers, the average daily cigarette consumption was calculated by multiplying the number of smoking days in a recent month by average cigarette consumption on smoking days, and then dividing by 30. Based on average daily cigarette consumption, smokers were classified as light (<10 cigarettes/d), moderate (≥10 to <20 cigarettes/d), or heavy (≥20 cigarettes/d) smokers. 1819)
Long-term smoking was defined with a cutoff of 20 years.20) The cumulative smoking exposure was determined in terms of pack-years by multiplying the number of years smoked by the number of packs smoked per day and were classified into groups of ≤10, >10 to ≤20, and >20 pack-years. Smoking cessation duration of former smokers was defined by subtracting the age at smoking initiation and past smoking duration from the age of the respondent. This study was approved by the institutional review board of Samsung Medical Center (IRB file no. SMC 2015-02-006).
3. Definition of Periodontal Disease
Maxillary and mandibular arches of the oral cavity were classified into 3 sextants each, making a total of 6 sextants (maxillary right posterior, maxillary anterior, maxillary left posterior, mandibular right posterior, mandibular anterior, and mandibular left posterior). Periodontal tissues of 10 index teeth were evaluated for bleeding status, the presence of dental plaque, and the presence of periodontal pockets with measurement of pocket depths, using a dental mirror and community periodontal index (CPI) probe. CPI scores were assigned as follows: 0 points for healthy periodontal tissue, 1 point for bleeding on probing only, 2 points when dental calculus was detected with probing, 3 points when a periodontal probing pocket depth of 4–5 mm had formed, and 4 points when a periodontal probing pocket depth of ≥6 mm had formed.15) The CPI reflects the health status of periodontal tissue, with a lower value indicating better periodontal status, and has been used for analysis of the dose-response relationship between smoking and periodontal disease in previous studies.2122) After assigning scores, the highest CPI score for the 6 sextants was selected. The highest CPI score of 3 or 4 points was defined as presence of periodontal disease, and the score of 0–2 points was defined as absence of periodontal disease.15)
To ensure reliability of the periodontal health survey, oral health examination research specialists (public health dentists) were trained twice per year, and agreement between the examiners and the gold standard of the Korean Academy of Dental Health was confirmed during training.15)
4. Statistical Analysis
The KNHANES data were subjected to a complex sampling design and were analyzed by applying stratum variance estimates, stratification variables, and sampling weights. Complex-samples χ2 tests were performed to determine the relationship between characteristics measured as categorical variables (sex, household income, education level, diabetes status, obesity status, alcohol drinking in the past year, current smoking status, and tooth brushing on the day before the survey) and periodontal disease.
The relationship between smoking status and periodontal disease, the dose-response relationship between smoking and periodontal disease, and the association between smoking cessation and periodontal disease were evaluated by using complex-samples multivariate logistic regression analysis. Sex, age, household income, education level, diabetes status, obesity status, alcohol drinking in the past year, and frequency of tooth brushing on the day before the survey were used as covariates for the analysis. These variables showed a statistically significant association with periodontal disease in a previous complexsamples χ2 test and were also reported to have a significant association with periodontal disease in previous studies.1112) All analyses were performed with a statistical significance level set at P<0.05 with 2-tailed tests using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
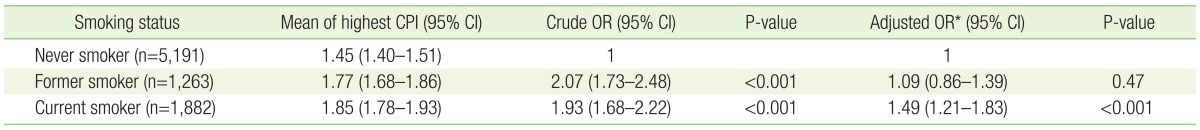
The prevalence of periodontal disease was 22.7% (1,893 individuals); 26.2% (1,055) were men and 15.5% (838) were women. The prevalence of periodontal disease was significantly higher for men, lower education level, lower income level, diabetes, obesity, lower frequency of tooth brushing, and alcohol consumption at least once per month in the last year. Current or former smokers had higher periodontal disease prevalence than never smokers (Table 1).
The association between current smoking status and periodontal disease was analyzed for all the subjects. The periodontal disease risk of current smokers was 1.49 times higher than that of never smokers (95% confidence interval [CI], 1.21–1.83); there was no statistically significant difference between never smokers and former smokers (Table 2).
The association between the degree of smoking exposure (average daily cigarette consumption, smoking duration, and cumulative smoking exposure) and periodontal disease was analyzed in current smokers compared with never smokers. There was no significant difference in periodontal disease risk between light smokers and never smokers, but moderate smokers had a 1.32 times higher risk of periodontal disease (95% CI, 1.01–1.74), and heavy smokers had a 2.33 times higher risk of periodontal disease (95% CI, 1.79–3.05). There was also no significant difference in periodontal disease risk between smokers of <20 years duration and never smokers, but long-term smokers of ≥20 years had a 1.87 times higher risk of periodontal disease (95% CI, 1.46–2.40). There was also no significant difference in periodontal disease risk between smokers of ≤10 pack-years and never smokers, whereas those with >10 to ≤20 pack-years of cumulative smoking exposure had a 1.83 times higher risk of periodontal disease (95% CI, 1.33–2.51), and the periodontal disease risk of those with >20 pack-years was increased 2.33-fold (95% CI, 1.75–3.11) (Table 3).
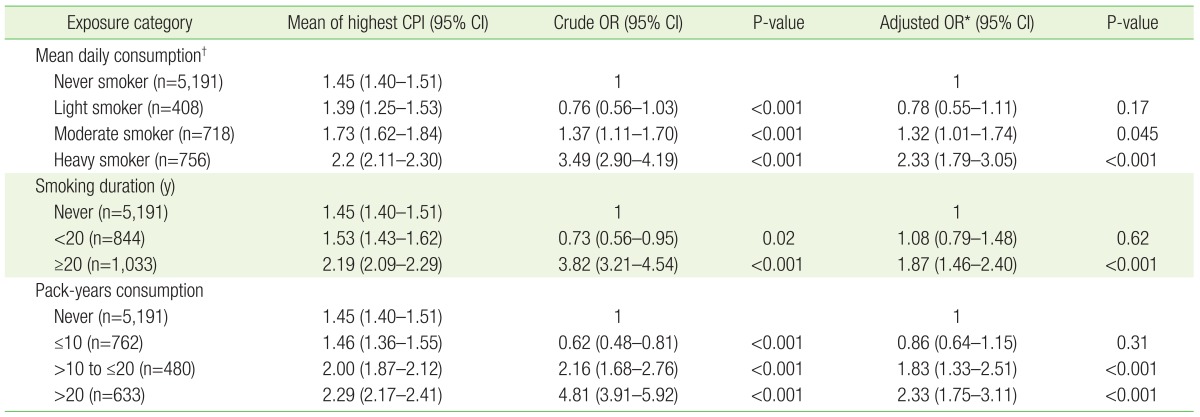
The association between smoking exposure categories for current smokers and periodontal disease compared with never smokers
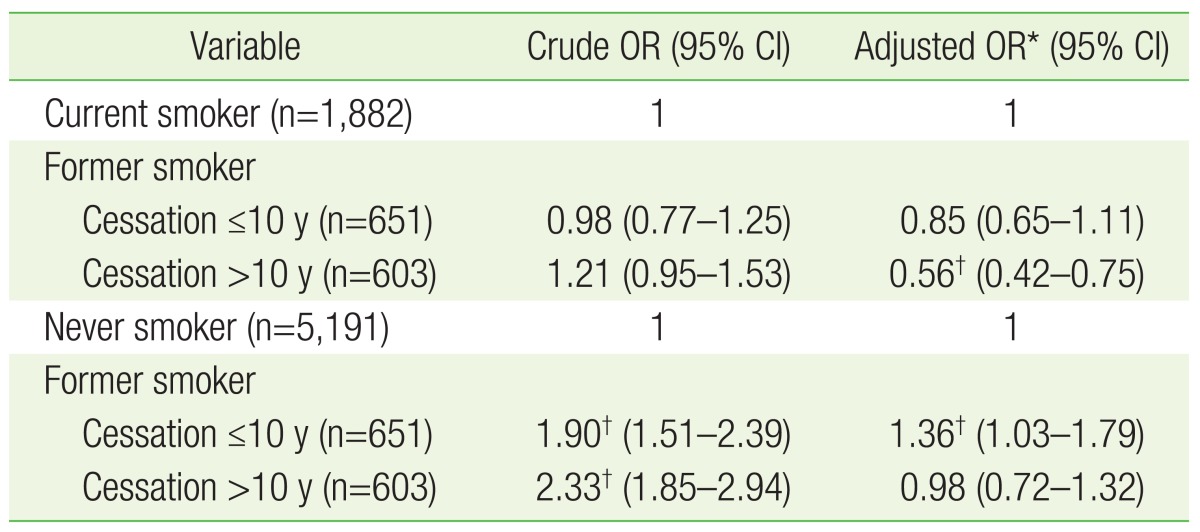
For the association between smoking cessation and periodontal disease, former smokers with a cessation duration of ≤10 years showed no significant difference in periodontal disease risk compared with current smokers; former smokers with a cessation duration of >10 years had a 0.56 times lower risk of periodontal disease than current smokers (95% CI, 0.42–0.75). Former smokers with a cessation duration of ≤10 years had a 1.36 times higher risk of periodontal disease than never smokers (95% CI, 1.03–1.79), whereas former smokers with a cessation duration of >10 years showed no statistically significant difference in periodontal disease risk compared with never smokers (Table 4).
DISCUSSION
The present study identified an increased risk of periodontal disease for current smokers compared with never smokers. In current smokers, the average daily cigarette consumption and cumulative exposure measured by cigarette amounts and smoking duration showed an association with periodontal disease. In addition, average daily cigarette consumption and cumulative smoking exposure showed a partial dose-response relationship with periodontal disease.
Former smokers, at >10 years after cessation, had a reduced periodontal disease risk compared with current smokers and showed no statistically significant difference compared with never smokers. This is consistent with the US National Health and Nutrition Examination Survey III (NHANES III), which also reported that former smokers who had quit ≥11 years previously showed no statistically significant difference in periodontitis risk compared with never smokers.23)
Periodontal disease risk in former smokers was similar to that of never smokers. This is consistent with the Japanese National Nutrition Survey and the Survey of Dental Diseases, which also showed no significant difference in periodontitis risk between former smokers and never smokers in adults aged ≥40 years.24) In contrast, periodontitis risk was 1.68 times higher in former smokers than in never smokers in the US NHANES III.23)
In addition, light smokers (<10 cigarettes/d) had no increase in periodontal disease risk compared with never smokers in the present study. Kye and Han25) evaluated 199 patients who visited a dentist in a university hospital in Korea and showed no significant difference in periodontal pocket depth between never smokers and light smokers (<10 cigarettes per day). In contrast, in the US NHANES III, smokers of ≤9 cigarettes smoked per day had a 2.79 times higher periodontitis risk compared with never smokers.23) In a Japanese longitudinal study of 1,332 men aged 30–59 years with daily cigarette consumption categorized as 1–19, 20, or ≥21 cigarettes per day, as daily cigarette consumption increased, periodontal disease also increased compared with that in never smokers; however, because they did not include a category of <10 cigarettes per day, no comparison was made between never smokers and light smokers.21) The reasons for the discrepancies in study results are as follows: first, the US NHANES III included only sex, age, race, education level, and income level as covariates,23) and the study of 30- to 59-year-old Japanese men21) adjusted only for age and alcohol drinking, without considering covariates such as diabetes and obesity, which were included in the present study. Second, it is possible that the effect of smoking on periodontal tissue is dependent on race. Heavy smokers (≥20 cigarettes/d) in the present study had a 2.33 times higher periodontal disease risk than never smokers, whereas smokers of ≥20 cigarettes per day had at least a 5 times higher periodontitis risk than never smokers in the US NHANES III.23)
Using the Korean National Oral Health Survey data from 2003, Park et al.26) tried to evaluate the dose-response relationship. Adults aged 50–59 years old who smoked ≥10 cigarettes per day had a higher frequency of sextants with periodontal pockets >3.5 mm in size than those who smoked <10 cigarettes, and 40- to 49-year-old adults who had been smoking for 15 years or longer had a higher frequency of sextants with periodontal pockets >3.5 mm. However, a dose-response relationship between smoking and periodontal pocket formation was not clearly demonstrated. In addition, Han et al.27) evaluated adults 18 years or older using the Korean National Oral Health Survey data from 2006 and reported an association only between cigarette consumption per year and periodontitis. The study by Kye and Han25) evaluating 199 patients who visited a dentist in a university hospital in Korea, which may not be representative of the Korean population.
However, the present study evaluated not only the effect of current smoking status on periodontal disease but also the dose-response relationships for average daily cigarette consumption, smoking duration, and cumulative smoking exposure using a nationwide survey, and the finding of a partial dose-response relationship makes this study significant.
The present study has several limitations. First, because it was a cross-sectional study, we could not determine whether the exposure to smoking preceded the onset of disease. In addition, we were unable to distinguish between existing periodontal disease and new periodontal disease caused by smoking. Second, the effects of comorbidity, except diabetes and obesity, or drugs that could affect periodontal disease may not have been reflected. Third, because self-administered survey data on smoking were used, the accuracy of measurement may be low. However, considering that current smokers are likely to report that they never smoked or were former smokers,28) it is highly probable that the association with periodontal disease was underestimated, rather than overestimated.
In conclusion, current smoking is associated with a higher risk of periodontal disease compared with nonsmoking, and the degree of smoking exposure (average daily cigarette consumption, smoking duration, and cumulative smoking exposure) showed partial dose-response relationships with periodontal disease. In addition, continued smoking cessation by former smokers is associated with a reduction of the periodontal disease risk compared with current smokers.
ACKNOWLEDGMENTS
We would like to thank the Korea Centers for Disease Control and Prevention for the data obtained from the fifth Korea National Health and Nutrition Examination Survey (KNHANES V) 2010–2012.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.