Association between Sleep Duration and Impaired Fasting Glucose in Korean Adults: Results from the Korean National Health and Nutrition Examination Survey 2011–2012
Article information
Abstract
Background
Impaired fasting glucose (IFG) is an established risk factor for type 2 diabetes and cardiovascular disease. This study evaluated the relationship between sleep duration and IFG.
Methods
This cross-sectional study included 14,925 Korean adults (5,868 men and 9,057 women) ≥19 years of age who participated in the Korean National Health and Nutrition Examination Survey between 2011 and 2012. Blood glucose levels were measured after at least eight hours of fasting. Study subjects were categorized into three groups based on self-reported sleep duration (<7, 7–8, or >8 h/d). IFG was diagnosed according to recommendations American Diabetes Association guidelines. Multiple logistic regression analysis was performed with adjustment for covariates.
Results
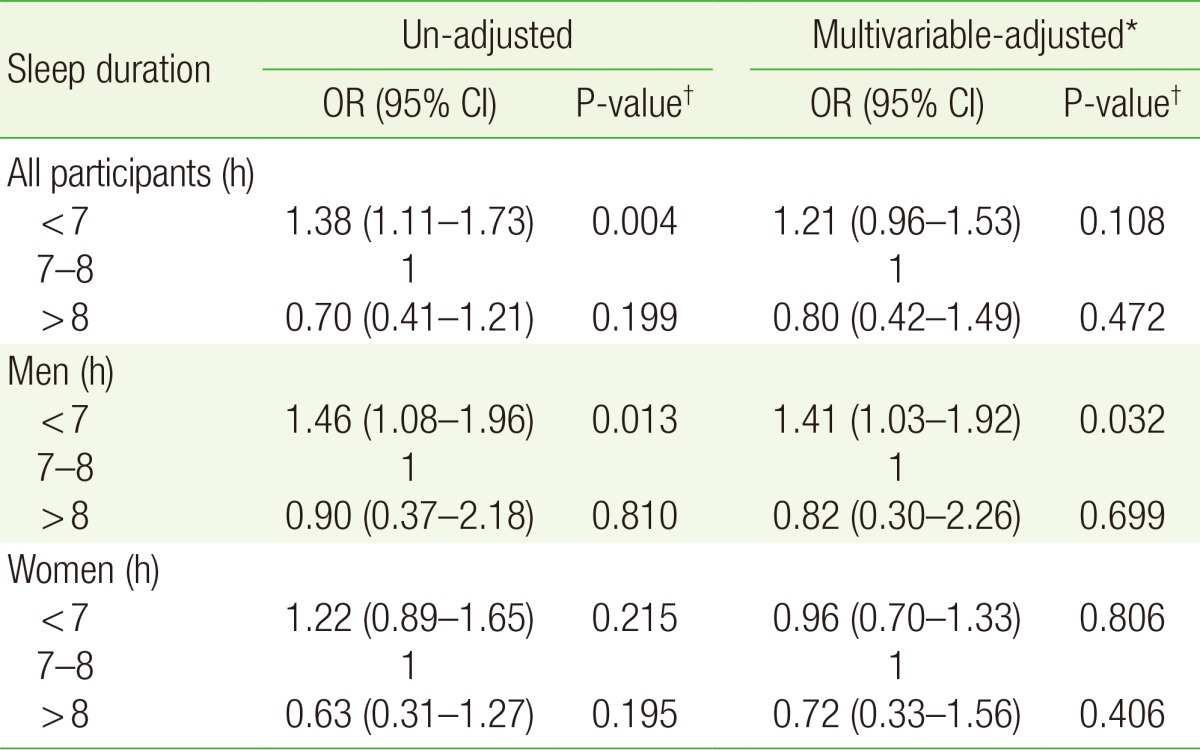
In men, short sleep duration (<7 hours) was associated with increased risk of IFG (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.08 to 1.96) compared to adequate sleep duration (7–8 hours), whereas long sleep duration (>8 hours) was not associated with risk of IFG (OR, 0.90; 95% CI, 0.37 to 2.18). In women, sleep duration was not associated with risk of IFG.
Conclusion
The association between sleep duration and IFG differed by sex; sleep deprivation, was associated with increased risk of IFG, especially in men.
INTRODUCTION
The proportion of people with type 2 diabetes has increased in recent decades, along with population aging, urbanization, obesity, and physical inactivity. The number of diabetic patients worldwide is expected to increase from 171 million in 2000 to 366 million by 2030.1) In Korea, the prevalence of diabetes has increased by six- to seven-fold, from 1.5% to 9.9%, in the past 40 years.2) Diabetes and its associated complications such as nephropathy, neuropathy, retinopathy, and coronary and cerebral artery diseases have become a leading cause of chronic morbidity, mortality, and increased medical costs.3)
Prediabetes, including impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), is a non-diabetic hyperglycemic status that does not fulfill the diagnostic criteria for diabetes, but which has clinical significance as a strong risk factor for type 2 diabetes.4) Without specific intervention, the annual incidence rate of diabetes was 11% over a six-year period in a cohort study of Chinese adults with IFG.5) The prediabetic state is also associated with increased cardiovascular disease and associated mortality. The risk of cardiovascular disease was more than 50% higher in prediabetes patients than in those with normal glucose tolerance, and was more evident in young adults.6) These reports suggest the importance of controlling prediabetes, likely through lifestyle modification.4)
Sleep plays an important role in the regulation of glucose metabolism, appetite, and endocrine and immune function.7) Inadequate sleep has been associated with obesity,8) insulin resistance,9) type 2 diabetes,10) metabolic syndrome,11) hypertension,8) cardiovascular diseases,8) and increased mortality.12) Prediabetes has also been suggested to be associated with sleep duration.1314)
However, it is uncertain whether sleep deprivation alone or both short and oversleeping are associated with prediabetes. In addition, research on the relationship between sleep duration and prediabetes is scarce in Korea, where chronic sleep deprivation is increasingly common.15)
In this context, we conducted a cross-sectional study to evaluate the relationship between sleep duration and IFG in adult Koreans using data from the fifth Korean National Health and Nutrition Examination Survey (KNHANES V).
METHODS
1. Study Subjects
This study used data from the second and the third year (2011 and 2012) of the KNHANES V. The KNHANES is a nationwide cross-sectional survey designed to assess the general health and nutritional status of representative samples of Koreans, supported by the Korea Centers for Disease Control and Prevention.16) This survey consists of health interview, health examination, and nutrition surveys.16) A total of 40,750 individuals participated in the second and the third year of the KNHANES V. Among them, 25,825 were excluded for the following reasons: <19 years of age (9,012 people); missing data on sleep duration, fasting plasma glucose level, glycated hemoglobin (HbA1C), blood pressure, height, weight, waist circumference, or other covariates (5,471 people); diabetes mellitus defined as use of insulin or hypoglycemic agents, a fasting plasma glucose level of 126 mg/dL or higher, or HbA1C level of 6.5% or higher (3,716 people); hypertension defined as blood pressure greater than or equal to 140/90 mm Hg, or regular use of antihypertensive medication (6,301 people); cardiovascular disease or stroke (271 people); cancer (439 people); or depression (615 people). Finally, a total of 14,925 subjects (5,868 men and 9,057 women) were included in this study.
This study was approved by the institutional review board of the Samsung Medical Center (IRB file No. 2014-11-052). The data used for this study did not include any identifiable personal information and were publically accessible for health research. Thus, the institutional review board waived informed consent.
2. Study Variables
We assessed sleep duration by asking "On average, how many hours a day do you usually sleep?" through a self-administered questionnaire. Based on their responses, we categorized study subjects into three groups according to their sleep duration: short (<7 h/d), adequate (7–8 h/d), and long (>8 h/d).
Venous blood was drawn after at least eight hours of fasting, and glucose levels were measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in a qualified central laboratory. IFG was defined as fasting glucose levels between 100–125 mg/dL, according to criteria from the American Diabetes Association.4)
Level of education, monthly family income, marital status, ever-smoking, current alcohol use, physical activity, stress recognition, and menopausal status were assessed using a self-administered questionnaire. Education level was categorized into two groups (<high school, or ≥high school). Monthly family income was categorized into four groups (≤1,000,000, 1,000,001–2,000,000, 2,000,001–3,000,000, or ≥3,000,001 Korean won). Marital status was categorized as ever-married or never-married. Ever-smoking was defined as having smoked at least 100 cigarettes in a lifetime. Current alcohol use was defined as at least one drink per month for the previous year. Regular physical activity was defined as participating in moderate activity for more than 30 min/d at least five days per week, or vigorous activity for more than 20 min/d at least three days per week. Stress recognition was assessed by asking "How much stress do you usually experience in your daily life?", and then categorized into two groups (high or low levels of stress). Menopausal status was categorized as premenopausal or postmenopausal states using a self-administered questionnaire. Body mass index (BMI, kg/m2) was calculated using measured weight and height. BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), or overweight/obese (≥23 kg/m2), according to recommendations from the Korean Society for the Study of Obesity.17)
3. Statistical Analysis
All analyses were conducted considering the complex design and sampling weight used for the KNHANES V. Differences in the distribution of baseline characteristics among categories of sleep duration were compared by the analysis of variance or chi-square tests. To evaluate the association between IFG and sleep duration, odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by multiple logistic regression analysis after adjusting for age, sex, and selected covariates (BMI, level of education, monthly family income, marital status, ever-smoking, current alcohol use, regular physical activity, stress recognition, and menopausal status). These covariates were selected because they were significantly associated with IFG or sleep duration in our study or in previous studies.101314181920) Statistical analysis was carried out using PASW SPSS Statistics for Windows ver. 18.0 (SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered statistically significant.
RESULTS
Table 1 shows the baseline characteristics of the study subjects according to sleep duration. In men, 41.4% and 5.4% were short- and long-duration sleepers, respectively. Only the distribution of regular physical activity significantly differed among the three sleep groups, and there was no difference in the distribution of other characteristics in men. In women, 37.7% and 7.5% were short- and long-duration sleepers, respectively. The distribution of age, education level, monthly family income, marital status, current alcohol use, stress level, and menopausal status were significantly different among women in the three sleep groups.
Figure 1 shows the IFG prevalence according to sleep duration categories by sex. The overall IFG prevalence was higher in men (17.6%) than in women (10.1%) (P<0.001). In men, the prevalence of IFG among short-, adequate-, and long-duration sleepers was 20.9%, 15.4%, and 14.0%, respectively. In women, the prevalence was 11.6%, 9.8%, and 6.4%, respectively. However, the prevalence of IFG was not significantly different between sleep duration categories both in men (P=0.074) and women (P=0.127).
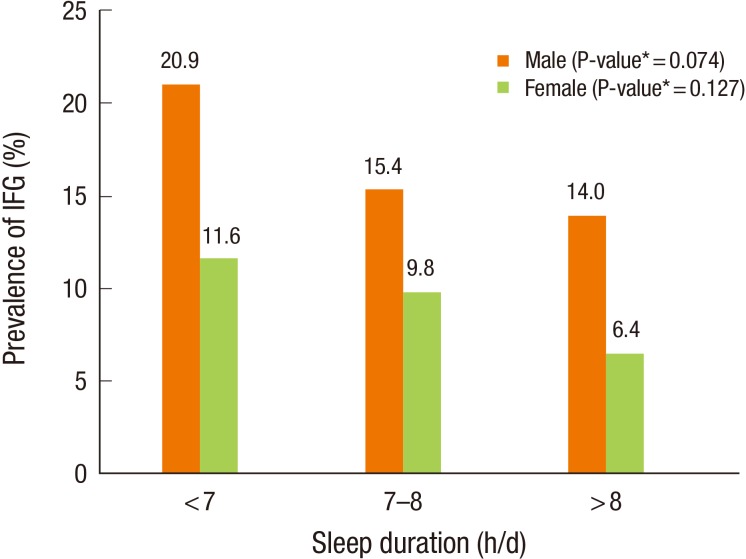
Prevalence of IFG according to sleep duration. IFG, impaired fasting glucose. *Obtained by chi-square test.
Table 2 shows the results of multiple logistic regression analysis conducted to evaluate the association between sleep duration and IFG. The un-adjusted analysis showed that short sleep duration in men (<7 hours) was associated with increased risk of IFG (OR, 1.46; 95% CI, 1.08 to 1.96) compared to 7–8 hours of sleep. This association persisted even after adjusting for covariates (OR, 1.41; 95% CI, 1.03 to 1.92). However, long sleep duration (>8 hours) was not associated with risk of IFG regardless of covariates adjustment. In women, shorter and longer sleep durations were not associated with risk of IFG, regardless of covariate adjustments. Subgroup analysis according to menopausal status (premenopausal or postmenopausal state) also showed no association between sleep duration and IFG, regardless of covariate adjustments.
DISCUSSION
In this large, cross-sectional study, we found that short sleep duration in men (<7 h/d) was associated with an increased risk of IFG, compared to adequate sleep duration (7–8 h/d). However, there was no association between sleep duration and IFG in women, and long sleep duration was not associated with increased risk of IFG.
These findings on the association between short sleep duration and IFG in men are consistent with those of previous studies. Short sleep duration has consistently been shown to be associated with increased risk of diabetes or prediabetes.101314181920) The Sleep Heart Health Study in American men and women found that self-reported short sleep duration (≤6 hours) was associated with higher risk of diabetes or IGT compared to 7–8 hours of sleep.10) A longitudinal (around six years of mean follow-up time) Quebec Family Study of 276 individuals aged 21 to 64 years found that an adjusted relative risk for the incidence of type 2 diabetes or IGT of 2.78 (95% CI, 1.61 to 4.12) in the short sleep group (≤6 hours), compared with the adequate sleep group (7–8 hours).19) Short sleep duration (≤5 hours) was also associated with increased prevalence of type 2 diabetes (OR, 2.40; 95% CI, 1.18 to 4.91) in Korean men without abdominal obesity.20) Lou et al.14) reported that short sleep duration (<6 hours) increased the risk of IFG in a Chinese population after adjusting for age, obesity, family history of diabetes, alcohol consumption, smoking, physical activity, and other diseases. A nested case-control study showed a three-fold increased risk of IFG (95% CI, 1.05 to 8.59) in short-duration sleepers (<6 hours) compared with adequate-duration sleepers (6–8 hours), after considering several diabetes risk factors.13) Engeda et al.18) reported short sleep duration (≤5 hours) to be associated with higher risk of clinically identified prediabetes.
There are several possible biological pathways through which sleep deprivation can lead to abnormal glucose regulation. First, sleep deprivation may lead to changes in the neurohormonal regulation of feeding behaviors.212223) A previous study examined the effect of sleep curtailment on mean levels of anorexigenic hormone (leptin) and appetite-stimulating hormone (ghrelin), hunger sense, and appetite. Mean leptin levels were 18% lower and mean ghrelin levels were 28% higher in participants who spent four hours in bed compared to their levels after 10 hours in bed. Short sleep duration (four hours) was also associated with a 24% and 23% increase in hunger and appetite, respectively, particularly for calorie-dense nutrients with high carbohydrate content such as sweets, salty snacks, and starchy food.21) The current study compared daily energy intake and carbohydrate intake among the three sleep groups; however, there was no difference in the distribution of daily energy and carbohydrate intake among the three groups in either men or women (Table 1). Sleep restriction to 5 h/d for one week resulted in significantly reduced insulin sensitivity in young healthy men.22) Moreover, short sleep duration may facilitate the development of insulin resistance and reduced glucose tolerance.23) Another possible pathway is the activation of the sympathetic nervous system. Among 11 young men with sleep restriction to 4 h/d for six nights, evening cortisol concentrations were elevated and the activity of the sympathetic nervous system was increased.24) The activation of inflammatory pathways may also play a role in the disruption of glucose metabolism. Reduced sleep is associated with increased tumor necrosis factor alpha, interleukin 6, and C-reactive protein.25) Furthermore, it is well known that inflammatory markers are strong predictors of type 2 diabetes.26)
Findings are inconsistent on the association between long sleep duration and diabetes or prediabetes.101419) The Sleep Heart Health Study found that self-reported long sleep duration (≥9 hours) was associated with a higher risk of diabetes or IGT compared to 7–8 hours of sleep.10) The Quebec Family Study found that adjusted relative risk for the incidence of type 2 diabetes or IGT was 2.54 (95% CI, 1.42 to 3.53) for long sleep group (≥9 hours).19) A Chinese study found that long sleep duration (>8 hours) increased the risk of IFG.14) However, some studies found no association between long sleep duration and the risk of diabetes or prediabetes, as shown in our study.131820) In addition, the biological mechanisms underlying the association between excessively long sleep duration and diabetes or prediabetes remain unknown.19)
IFG prevalence differs by sex, and men tend to have a higher rate of IFG than women,27) as found in our study. Compared to women, men are known to have a lower sleep efficiency and higher wakefulness during sleep.28) Men also tend to have a longer stage 1 sleep and shorter rapid eye movement sleep latency.28) Thus, the association between short sleep duration and IFG may be amplified in men. Given these differences in IFG prevalence and sleep characteristics, it seems necessary to evaluate the association between IFG and sleep duration separately in men and women. Moreover, menopausal status was selected as an additional covariate in women because the postmenopausal state is significantly associated with the presence of type 2 diabetes and prediabetes.29) We found that the association between IFG and sleep duration differs by sex. However, we could not compare the findings of our study to those of other studies because most previous studies did not separately evaluate the association by sex. Although one study reported that a significant association between limited sleep duration and metabolic syndrome in men, it did not report the relationship between sleep duration and IFG.11)
This study has several limitations. First, the cross-sectional study design prevented determination of the causal direction of the relationship between sleep duration and IFG. Second, sleep duration was assessed by self-report. Considering that assessment of sleep time by self-report was found to be moderately valid compared to measured sleep (r=0.45) in a study,30) self-reporting may result in a biased estimation of the association between short sleep duration and IFG. Third, we could not assess sleep quality in this study. Because poor sleep quality has been associated with the risk of diabetes or prediabetes,1418) it is necessary to evaluate the total effect of sleep, including quality as well as quantity, on the risk of IFG. Fourth, we could not consider the probable confounding effects of medical comorbidities, sedentary lifestyle, and dietary factors. Finally, we determined glycemic status based on a one-time measurement of fasting glucose concentration, which may have resulted in IFG misclassification.
In conclusion, the findings of this cross-sectional study of Korean adults suggest that short sleep duration rather than long sleep duration may increase the risk of IFG, although the association between short sleep duration and IFG was evident only in men.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01002705).
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.