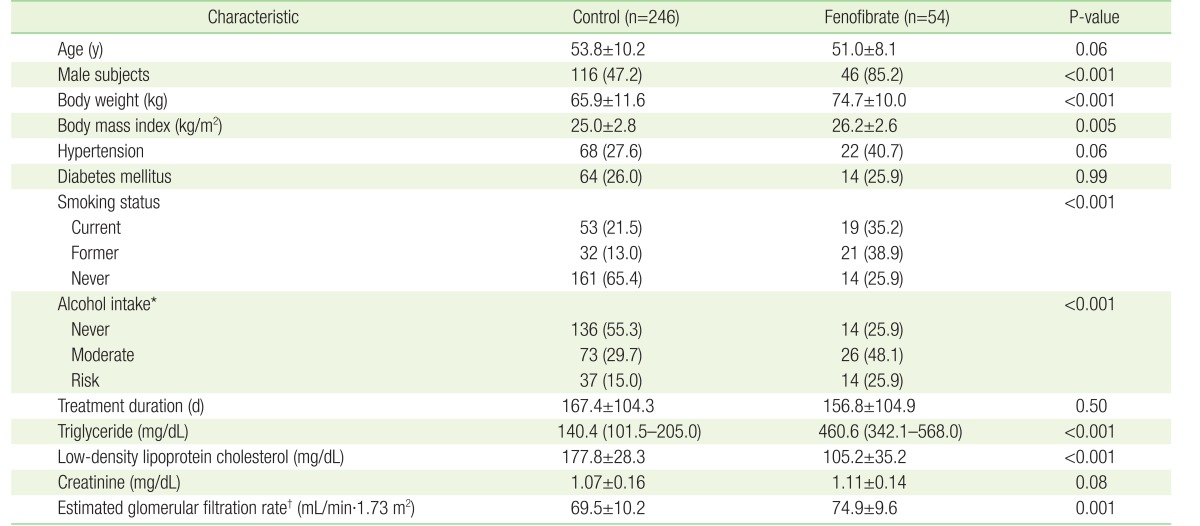
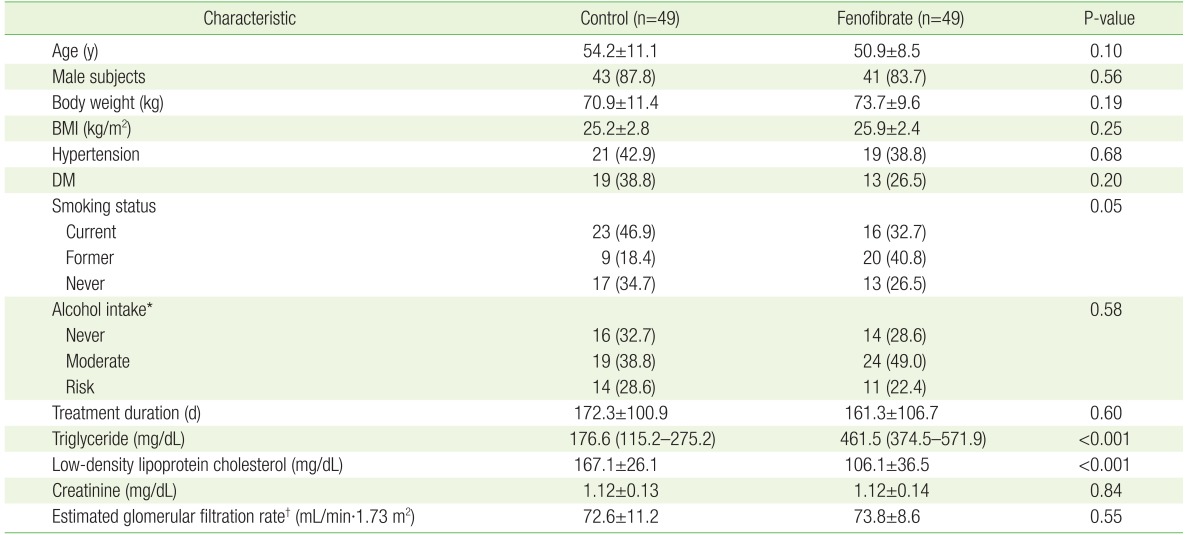
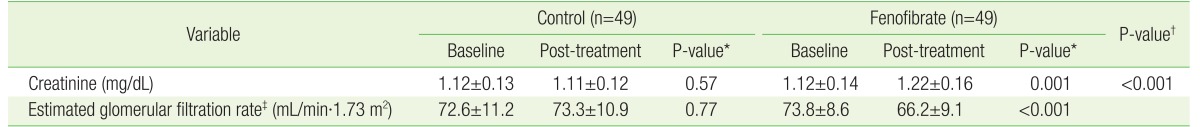
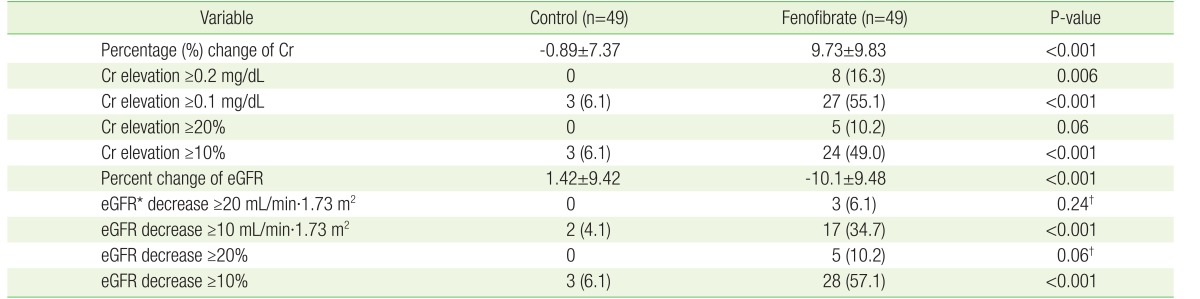
1. Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis 2010;210:35-40. PMID:
20005515.


2. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-1278. PMID:
16214597.


3. Cho JH, Choi YH, Hyeon CW, Kim KJ, Hyun S, Kwon JE, et al. Effect of fenofibrate therapy on blood creatinine levels in patients with hypertension and hypertriglyceridemia. J Lipid Atheroscler 2013;2:19-26.

4. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889-2934. PMID:
24239923.


5. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-3421. PMID:
12485966.


6. Han SH, Nicholls SJ, Sakuma I, Zhao D, Koh KK. Hypertriglyceridemia and cardiovascular diseases: revisited. Korean Circ J 2016;46:135-144. PMID:
27014342.



7. Abourbih S, Filion KB, Joseph L, Schiffrin EL, Rinfret S, Poirier P, et al. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am J Med 2009;122:962.e1-962.e8. PMID:
19698935.


8. Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol 2007;99:3C-18C.


9. Rossner S, Oro L. Fenofibrate therapy of hyperlipoproteinaemia: a dose-response study and a comparison with clofibrate. Atherosclerosis 1981;38:273-282. PMID:
7225166.


10. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849-1861. PMID:
16310551.


11. ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563-1574. PMID:
20228404.



12. Lageder H. Comparative double-blind investigation of bezafibrate and clofibrate in patients with primary hyperlipoproteinaemia. Wien Klin Wochenschr 1980;92:95-101. PMID:
6985560.

13. Dick TB, Marples J, Ledermann HM, Whittington J. Comparative study of once and 3-times daily regimens of bezafibrate in patients with primary hyperlipoproteinaemia. Curr Med Res Opin 1981;7:489-502. PMID:
7030635.


14. Attridge RL, Frei CR, Ryan L, Koeller J, Linn WD. Fenofibrate-associated nephrotoxicity: a review of current evidence. Am J Health Syst Pharm 2013;70:1219-1225. PMID:
23820458.


15. Hukkanen J, Puurunen J, Hyotylainen T, Savolainen MJ, Ruokonen A, Morin-Papunen L, et al. The effect of atorvastatin treatment on serum oxysterol concentrations and cytochrome P450 3A4 activity. Br J Clin Pharmacol 2015;80:473-479. PMID:
26095142.



16. Takazakura A, Sakurai M, Bando Y, Misu H, Takeshita Y, Kita Y, et al. Renoprotective effects of atorvastatin compared with pravastatin on progression of early diabetic nephropathy. J Diabetes Investig 2015;6:346-353.


17. Lee CH. Estimation of GFR. Korean J Med 2012;83:455-457.

18. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-161. PMID:
20925139.


19. Backes JM, Gibson CA, Ruisinger JF, Moriarty PM. Fibrates: what have we learned in the past 40 years. Pharmacotherapy 2007;27:412-424. PMID:
17316152.


20. Jacobson TA, Miller M, Schaefer EJ. Hypertriglyceridemia and cardiovascular risk reduction. Clin Ther 2007;29:763-777. PMID:
17697898.


21. Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ 2007;176:1113-1120. PMID:
17420495.



22. Angeles C, Lane BP, Miller F, Nord EP. Fenofibrate-associated reversible acute allograft dysfunction in 3 renal transplant recipients: biopsy evidence of tubular toxicity. Am J Kidney Dis 2004;44:543-550. PMID:
15332227.


23. Keating GM, Croom KF. Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs 2007;67:121-153. PMID:
17209672.


24. Remick J, Weintraub H, Setton R, Offenbacher J, Fisher E, Schwartzbard A. Fibrate therapy: an update. Cardiol Rev 2008;16:129-141. PMID:
18414184.


25. Broeders N, Knoop C, Antoine M, Tielemans C, Abramowicz D. Fibrate-induced increase in blood urea and creatinine: is gemfibrozil the only innocuous agent. Nephrol Dial Transplant 2000;15:1993-1999. PMID:
11096145.


26. Paul S, Mohan V. Fenofibrate can increase serum creatinine levels in renal insufficiency. J Assoc Physicians India 2006;54:337PMID:
16944623.

27. McQuade CR, Griego J, Anderson J, Pai AB. Elevated serum creatinine levels associated with fenofibrate therapy. Am J Health Syst Pharm 2008;65:138-141. PMID:
18192258.


28. Lipscombe J, Lewis GF, Cattran D, Bargman JM. Deterioration in renal function associated with fibrate therapy. Clin Nephrol 2001;55:39-44. PMID:
11200866.

29. Ritter JL, Nabulsi S. Fenofibrate-induced elevation in serum creatinine. Pharmacotherapy 2001;21:1145-1149. PMID:
11560205.


30. Ansquer JC, Dalton RN, Causse E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. Am J Kidney Dis 2008;51:904-913. PMID:
18501783.


31. Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron 2002;92:536-541. PMID:
12372935.


32. Attridge RL, Linn WD, Ryan L, Koeller J, Frei CR. Evaluation of the incidence and risk factors for development of fenofibrate-associated nephrotoxicity. J Clin Lipidol 2012;6:19-26. PMID:
22264570.


33. Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH, Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care 2010;33:215-220. PMID:
19846798.


34. Mychaleckyj JC, Craven T, Nayak U, Buse J, Crouse JR, Elam M, et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 2012;35:1008-1014. PMID:
22432114.



35. Patel P, Barkate H. Comparison of efficacy and safety of choline fenofibrate (fenofibric acid) to micronized fenofibrate in patients of mixed dyslipidemia: a randomized, open-label, multicenter clinical trial in Indian population. Indian J Endocrinol Metab 2016;20:67-71. PMID:
26904471.