 |
 |
- Search
| Korean J Fam Med > Volume 39(6); 2018 > Article |
|
Abstract
Background
Methods
Results
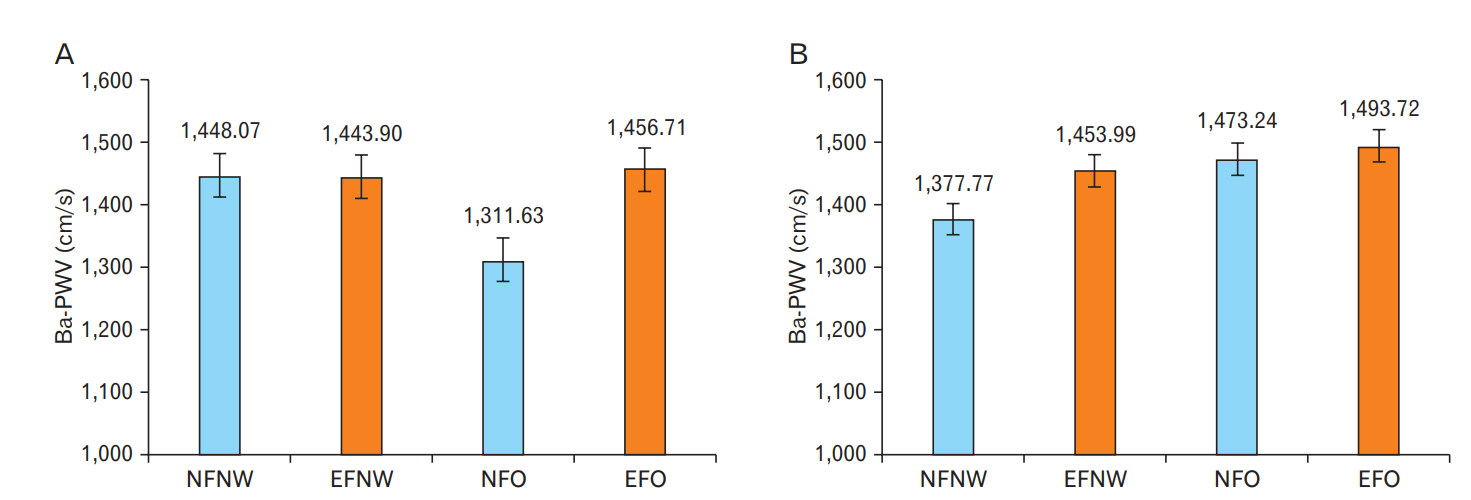
Figure.┬Ā1.
Table┬Ā1.
| Characteristic | NFNW (n=206) | EFNW (n=414) | NFO (n=21) | EFO (n=403) | P-value |
|---|---|---|---|---|---|
| Age (y) | 58.90┬▒6.30 | 58.92┬▒6.44 | 59.48┬▒7.87 | 58.24┬▒6.10 | 0.371 |
| Anthropometric data | |||||
| ŌĆāHeight (cm) | 170.81┬▒6.13b | 169.51┬▒5.31a | 171.28┬▒5.51ab | 169.81┬▒5.47a | 0.029 |
| ŌĆāWeight (kg) | 63.44┬▒6.93a | 67.61┬▒5.18b | 75.69┬▒4.15c | 77.47┬▒6.86c | <0.001 |
| ŌĆāpBF (%) | 18.30 (16.40, 19.50)a | 23.50 (22.10, 24.50)c | 19.10 (18.15, 20.00)b | 26.20 (24.50, 28.10)d | <0.001 |
| ŌĆāBMI (kg/m2) | 21.70 (20.50, 22.90)a | 23.60 (22.90, 24.20)b | 25.40 (25.10, 26.25)c | 26.40 (25.60, 27.70)d | <0.001 |
| ŌĆāMuscle mass (kg) | 48.52┬▒5.16a | 47.82┬▒3.61a | 57.07┬▒3.56c | 52.53┬▒4.59b | <0.001 |
| ŌĆāMuscle ratio (%) | 75.92 (74.68, 77.83)d | 70.76 (69.74, 72.05)b | 75.10 (74.19, 76.03)c | 68.01 (66.13, 69.73)a | <0.001 |
| ŌĆāSkeletal muscle mass (kg) | 24.43┬▒4.61b | 23.41┬▒3.05a | 34.00┬▒2.23d | 26.92┬▒4.69c | <0.001 |
| ŌĆāSkeletal muscle ratio (%) | 38.36 (35.24, 41.07)b | 34.89 (32.39, 37.13)a | 44.52 (43.03, 47.17)c | 33.81 (30.76, 39.11)a | <0.001 |
| ŌĆāSystolic BP (mm Hg) | 119.32┬▒15.57a | 123.36┬▒15.45b | 124.29┬▒14.07abc | 128.65┬▒15.44c | <0.001 |
| ŌĆāDiastolic BP (mm Hg) | 73.94┬▒9.71a | 76.67┬▒10.39b | 77.19┬▒10.29abc | 80.20┬▒10.24c | <0.001 |
| Biochemical markers | |||||
| ŌĆāTotal cholesterol (mg/dL) | 190.55┬▒32.44a | 202.31┬▒35.1c | 197.10┬▒38.37abc | 197.40┬▒37.54b | 0.002 |
| ŌĆāTriglyceride (mg/dL) | 79.00 (61.00, 114.75)a | 105.50 (76.00, 151.00)b | 105.00 (86.00, 182.00)bc | 118.00 (85.00, 164.00)c | <0.001 |
| ŌĆāHigh-density lipoprotein (mg/dL) | 57.38┬▒16.75c | 51.98┬▒12.73b | 48.24┬▒9.20ab | 49.05┬▒12.14a | <0.001 |
| ŌĆāLow-density lipoprotein (mg/dL) | 123.34┬▒29.92a | 136.32┬▒31.80b | 130.14┬▒35.10ab | 132.15┬▒34.63b | <0.001 |
| ŌĆāFasting glucose (mg/dL) | 92.96┬▒21.37a | 95.91┬▒25.20a | 95.10┬▒32.43ab | 101.59┬▒30.12b | 0.001 |
| ŌĆāFasting insulin (mIU/L) | 4.09 (3.23, 4.94)a | 4.95 (3.88, 6.65)b | 5.01 (4.22, 7.23)bc | 5.56 (4.45, 8.70)c | <0.001 |
| ŌĆāHomeostatic model assessment of insulin resistance | 0.91 (0.66, 1.10)a | 1.15 (0.85, 1.61)b | 1.11 (0.96, 1.47)b | 1.37 (1.01, 2.22)b | <0.001 |
| ŌĆāC-reactive protein (mg/dL) | 0.04 (0.02, 0.09) | 0.06 (0.03, 0.11) | 0.08 (0.04, 0.21) | 0.07 (0.03, 0.13) | 0.472 |
| ŌĆāBa-PWV, right (cm/s) | 1,415.68┬▒228.10b | 1,424.85┬▒203.28b | 1,318.76┬▒223.69a | 1,424.95┬▒206.57b | 0.146 |
| ŌĆāBa-PWV, left (cm/s) | 1,425.48┬▒216.67b | 1,433.69┬▒191.03b | 1,320.90┬▒174.11a | 1,428.79┬▒203.88b | 0.097 |
| ŌĆāMean ba-PWV (cm/s) | 1,420.58┬▒216.99b | 1,429.27┬▒193.58b | 1,319.83┬▒198.04a | 1,427.06┬▒199.93b | 0.109 |
| Disease history* | |||||
| ŌĆāDiabetes mellitus | 20 (9.7) | 33 (8.0) | 4 (19.0) | 51 (12.7) | 0.084 |
| ŌĆāHypertension | 36 (17.5) | 89 (21.5) | 4 (19.0) | 142 (35.2) | <0.001 |
| ŌĆāDyslipidemia | 20 (9.7) | 56 (13.5) | 2 (9.5) | 71 (17.6) | 0.051 |
| SmokingŌĆĀ | 0.132 | ||||
| ŌĆāNon-smokers | 57 (27.7) | 103 (24.9) | 9 (42.9) | 91 (22.6) | |
| ŌĆāSmokers | 149 (72.3) | 311 (75.1) | 12 (57.1) | 312 (77.4) | |
| DrinkingŌĆĪ | 96 (46.6) | 224 (54.1) | 8 (38.1) | 239 (59.3) | 0.010 |
| Exercise┬¦ | 40 (19.4) | 113 (27.3) | 8 (38.1) | 94 (23.3) | 0.071 |
Values are presented as mean┬▒standard deviation, median (1st quartile, 3rd quartile), or number (%). P-value was calculated using an ANOVA, Kruskal-Wallis, or Žć2 test.
Superscript (a, b, c, d): different superscript show post-hoc results using the least significant difference method in the ANOVA.
BMI, body mass index; pBF, body fat percentage; NF, normal fat; NW, normal weight; EF, excessive fat; O, obese; BP, blood pressure; Ba-PWV, brachial-ankle pulse wave velocity; ANOVA, analysis of variance.
Table┬Ā2.
| Characteristic | NFNW (n=449) | EFNW (n=46) | NFO (n=43) | EFO (n=118) | P-value |
|---|---|---|---|---|---|
| Age (y) | 58.61┬▒6.27a | 63.54┬▒7.32b | 57.14┬▒5.09a | 61.38┬▒7.17b | <0.001 |
| Anthropometric data | |||||
| ŌĆāHeight (cm) | 158.19┬▒4.82b | 155.82┬▒5.56a | 158.07┬▒3.69b | 155.64┬▒4.83a | <0.001 |
| ŌĆāWeight (kg) | 54.81┬▒4.88a | 59.03┬▒4.09b | 64.90┬▒4.90c | 66.26┬▒6.61c | <0.001 |
| ŌĆāpBF (%) | 28.70 (26.75, 30.80)a | 33.95 (33.60, 34.93)c | 32.30 (31.60, 32.90)b | 35.70 (34.55, 37.43)d | <0.001 |
| ŌĆāBMI (kg/m2) | 21.90 (20.75, 23.00)a | 24.30 (24.00, 24.70)b | 25.50 (25.20, 26.50)c | 26.70 (25.88, 28.23)d | <0.001 |
| ŌĆāMuscle mass (kg) | 36.00┬▒2.76a | 35.46┬▒2.62a | 40.32┬▒4.30c | 38.46┬▒3.14b | <0.001 |
| ŌĆāMuscle ratio (%) | 65.65 (63.54, 67.53)d | 60.46 (59.42, 60.68)b | 61.98 (61.47, 62.52)c | 58.61 (56.91, 59.78)a | <0.001 |
| ŌĆāSkeletal muscle mass (kg) | 18.54┬▒3.07b | 17.00┬▒2.83a | 25.31┬▒4.27c | 18.99┬▒3.79b | <0.001 |
| ŌĆāSkeletal muscle ratio (%) | 33.85 (30.82, 36.59)b | 30.14 (25.48, 31.82)a | 38.85 (37.04, 41.22)c | 26.84 (24.46, 33.32)a | <0.001 |
| ŌĆāSystolic BP (mm Hg) | 118.13┬▒16.55a | 125.89┬▒19.71b | 126.77┬▒14.10b | 130.32┬▒17.36b | <0.001 |
| ŌĆāDiastolic BP (mm Hg) | 72.13┬▒10.20a | 74.35┬▒10.95ab | 77.88┬▒8.60bc | 78.53┬▒10.12c | <0.001 |
| Biochemical markers | |||||
| ŌĆāTotal cholesterol (mg/dL) | 203.27┬▒38.86 | 205.41┬▒33.04 | 200.67┬▒34.58 | 207.36┬▒35.16 | 0.679 |
| ŌĆāTriglyceride (mg/dL) | 72.00 (54.00, 100.00)a | 86.50 (60.25, 109.25)ab | 99.00 (59.00, 138.00)ab | 103.50 (68.75, 146.25)b | <0.001 |
| ŌĆāHigh-density lipoprotein (mg/dL) | 63.39┬▒16.50b | 59.72┬▒11.86ab | 55.49┬▒9.85a | 56.61┬▒12.58a | <0.001 |
| ŌĆāLow-density lipoprotein (mg/dL) | 131.40┬▒36.58 | 137.89┬▒29.76 | 133.98┬▒31.20 | 138.30┬▒33.47 | 0.217 |
| ŌĆāFasting glucose (mg/dL) | 88.48┬▒18.45a | 95.93┬▒35.43b | 94.81┬▒15.25ab | 94.76┬▒20.27b | 0.002 |
| ŌĆāFasting insulin (mIU/L) | 4.48 (3.46, 6.11)a | 5.61 (4.75, 8.62)b | 5.76 (3.83, 9.55)b | 5.24 (4.23, 8.20)b | <0.001 |
| ŌĆāHomeostatic model assessment of insulin resistance | 0.96 (0.71, 1.32)a | 1.38 (1.05, 1.94)b | 1.39 (0.91, 2.18)b | 1.22 (0.91, 1.91)b | <0.001 |
| ŌĆāC-reactive protein (mg/dL) | 0.04 (0.02, 0.07) | 0.07 (0.03, 0.14) | 0.05 (0.03, 0.11) | 0.09 (0.04, 0.19) | 0.864 |
| ŌĆāBa-PWV, right (cm/s) | 1,370.73┬▒229.90a | 1,472.70┬▒266.20b | 1,401.09┬▒211.99ab | 1,472.42┬▒248.67b | <0.001 |
| ŌĆāBa-PWV, left (cm/s) | 1,378.82┬▒217.08a | 1,470.07┬▒246.05b | 1,417.00┬▒187.81ab | 1,469.46┬▒231.70b | <0.001 |
| ŌĆāMean ba-PWV (cm/s) | 1,374.78┬▒220.36a | 1,471.38┬▒253.69b | 1,409.05┬▒196.22ab | 1,470.94┬▒236.39b | <0.001 |
| Disease history* | |||||
| ŌĆāDiabetes mellitus | 19 (4.2) | 5 (10.9) | 4 (9.3) | 11 (9.3) | 0.032 |
| ŌĆāHypertension | 80 (17.8) | 16 (34.8) | 11 (25.6) | 44 (37.3) | <0.001 |
| ŌĆāDyslipidemia | 67 (14.9) | 12 (26.1) | 7 (16.3) | 24 (20.3) | 0.169 |
| SmokingŌĆĀ | 0.235 | ||||
| ŌĆāNon-smokers | 437 (97.3) | 43 (93.5) | 42 (97.7) | 112 (94.9) | |
| ŌĆāSmokers | 12 (2.7) | 3 (6.5) | 1 (2.3) | 6 (5.1) | |
| DrinkingŌĆĪ | 66 (14.7) | 10 (21.7) | 7 (16.3) | 17 (14.4) | 0.636 |
| Exercise┬¦ | 111 (24.7) | 10 (21.7) | 8 (18.6) | 30 (25.4) | 0.790 |
Values are presented as mean┬▒standard deviation, median (1st quartile, 3rd quartile), or number (%). P-value was calculated using an ANOVA, Kruskal-Wallis, or Žć2 test.
Superscript (a, b, c, d): different superscript show post-hoc results using the least significant difference method in the ANOVA.
BMI, body mass index; pBF, body fat percentage; NF, normal fat; NW, normal weight; EF, excessive fat; O, obese; BP, blood pressure; Ba-PWV, brachial-ankle pulse wave velocity; ANOVA, analysis of variance.
Table┬Ā3.
| Variable |
Correlation coefficient, r (P-value) |
|
|---|---|---|
| Men | Women | |
| Body fat percentage (%) | 0.095* (0.002) | 0.175* (<0.001) |
| Body mass index (kg/m2) | 0.002* (0.959) | 0.226* (<0.001) |
| Muscle mass (kg) | -0.143ŌĆĀ (<0.001) | -0.131ŌĆĀ (<0.001) |
| Muscle ratio (%) | -0.093* (0.003) | -0.229* (<0.001) |
| Skeletal muscle mass (kg) | -0.127ŌĆĀ (<0.001) | -0.002ŌĆĀ (0.959) |
| Skeletal muscle ratio (%) | -0.100* (0.001) | -0.003* (0.947) |
Table┬Ā4.
| Variable | NFNW | EFNW | NFO | EFO | P-value | |
|---|---|---|---|---|---|---|
| Men (n=1,044) | No. of participants | 206 | 414 | 21 | 403 | |
| Mean ba-PWV | 1,420.58┬▒216.99b | 1,429.27┬▒193.58b | 1,319.83┬▒198.04a | 1,427.06┬▒199.93b | 0.109 | |
| Age adjusted* | 1,417.60 (12.87)b | 1,426.07 (9.08)b | 1,309.64 (40.30)a | 1,432.41 (9.21)b | 0.028 | |
| Social adjustedŌĆĀ | 1,427.12 (22.08)b | 1,414.38 (14.71)b | 1,277.10 (45.78)a | 1,434.16 (13.18)b | 0.011 | |
| Medical adjustedŌĆĪ | 1,448.07 (19.13)b | 1,443.90 (16.02)b | 1,311.63 (42.71)a | 1,456.71 (13.50)b | 0.015 | |
| Women (n=656) | No. of participants | 449 | 46 | 43 | 118 | |
| Mean ba-PWV | 1,374.78┬▒220.36a | 1,471.38┬▒253.69b | 1,409.05┬▒196.22ab | 1,470.94┬▒236.39b | <0.001 | |
| Age adjusted* | 1,388.88 (8.94)a | 1,392.39 (28.21)ab | 1,451.01 (28.86)b | 1,432.78 (17.51)b | 0.041 | |
| Social adjustedŌĆĀ | 1,378.84 (39.32) | 1,450.65 (51.84) | 1,463.46 (58.12) | 1,446.56 (42.06) | 0.509 | |
| Medical adjustedŌĆĪ | 1,377.77 (28.58)a | 1,453.99 (37.97)ab | 1,473.24 (40.17)ab | 1,493.72 (32.11)b | 0.040 | |
Values are presented as mean┬▒standard deviation or mean (standard error). P-value was calculated using an ANOVA or analysis of covariance, adjusting for variables as indicated. Superscript (a, b, c, d): mean values with different superscript are significantly different (P<0.05, ANOVA followed by the least significant difference method).
Ba-PWV, brachial-ankle pulse wave velocity; NF, normal fat; NW, normal weight; EF, excessive fat; O, obese; ANOVA, analysis of variance.
REFERENCES
- TOOLS







