 |
 |
- Search
| Korean J Fam Med > Volume 40(2); 2019 > Article |
|
Abstract
Figure. 1.
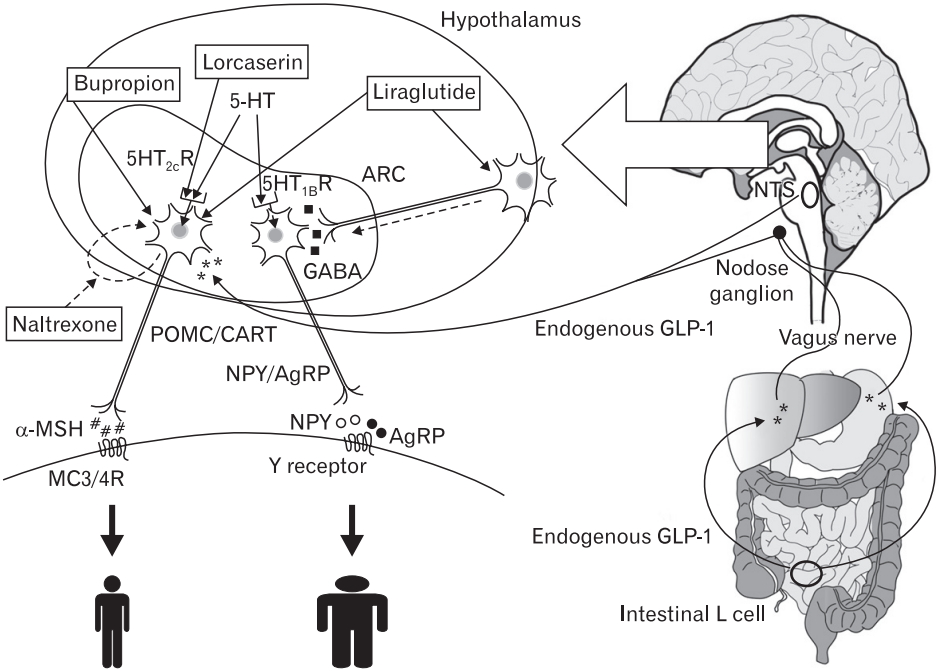
Table 1.
Controlled substance schedules are divided into five classes according to the medical usage and the relative abuse potential of each substance by the US Drug Enforcement Administration. For the definition of schedules and example drugs, refer to reference number 3.[3]
MFDS, Korean Ministry of Food and Drug Safety; NA, not applicable; GLP-1, glucagon-like peptide 1.
Table 2.
| Author (year) | Study duration (wk) | Group | No. | Completion | Age (y) | Initial bodyweight (kg) | Initial body mass index (kg/m2) | Mean weight loss (%) |
|---|---|---|---|---|---|---|---|---|
| Smith et al. [25] (2010) | 52 | Lorcaserin 20 mg | 1,595 | 883 | 43.8 | 100.4±0.4 | 36.2±0.1 | -5.8 |
| Placebo | 1,587 | 716 | 44.4 | 99.7±0.4 | 36.2±0.1 | -2.2 | ||
| Fidler et al. [26] (2011) | 52 | Lorcaserin 20 mg | 1,602 | 917 | 43.8 | 100.1±15.6 | 36.0±4.3 | -5.8 |
| Lorcaserin 10 mg | 801 | 473 | 43.8 | 99.8±16.6 | 35.8±4.3 | -4.7 | ||
| Placebo | 1,601 | 834 | 43.7 | 100.5±16.2 | 35.9±4.1 | -2.8 | ||
| O’Neil et al. [27] (2012)* | 52 | Lorcaserin 20 mg | 256 | 169 | 53.2 | 103.7±17.0 | 36.1±4.5 | -4.5 |
| Lorcaserin 10 mg | 95 | 75 | 53.1 | 106.0±19.4 | 36.1±4.8 | -5.0 | ||
| Placebo | 253 | 157 | 52.0 | 102.6±18.1 | 35.9±4.5 | -1.5 | ||
| Greenway et al. [41] (2010) | 56 | Naltrexone 32 mg+Bupropion 360 mg | 583 | 296 | 44.4 | 99.7±15.9 | 36.1±4.4 | -6.1 |
| Naltrexone 16 mg+Bupropion 360 mg | 578 | 284 | 44.4 | 99.5±14.8 | 36.2±4.3 | -5.0 | ||
| Placebo | 581 | 290 | 43.7 | 99.5±14.3 | 36.2±4.0 | -1.3 | ||
| Wadden et al. [42] (2011)† | 56 | Naltrexone 32 mg+Bupropion 360 mg | 591 | 342 | 45.9 | 100.2±15.4 | 36.3±4.2 | -9.3 |
| Placebo | 202 | 118 | 45.6 | 101.9±15.0 | 37.0±4.2 | -5.1 | ||
| Apovian et al. [43] (2013) | 56 | Naltrexone 32 mg+Bupropion 360 mg | 1,001 | 538 | 44.3 | 100.3±16.6 | 36.2±4.5 | -6.4 |
| Placebo | 495 | 267 | 44.4 | 99.2±15.9 | 36.1±4.3 | -1.2 | ||
| Hollander et al. [44] (2013)* | 56 | Naltrexone 32 mg+Bupropion 360 mg | 335 | 175 | 54.0 | 104.2±18.9 | 36.4±4.8 | -5.0 |
| Placebo | 170 | 100 | 53.5 | 105.1±17.0 | 36.4±4.5 | -1.8 |
Table 3.
| Author (year) | Study duration (wk) | Group | No. | Completion | Age (y) | Initial body weight (kg) | Initial BMI (kg/m2) | Mean weight loss (%) |
|---|---|---|---|---|---|---|---|---|
| Wadden et al. [28] (2013)* | 56 | Liraglutide 3.0 mg | 212 | 159 | 45.9 | 100.4±20.8 | 36.0±5.9 | -6.2 |
| Placebo | 210 | 146 | 46.5 | 98.7±21.2 | 35.2±5.9 | 0.2 | ||
| Pi-Sunyer et al. [29] (2015) | 56 | Liraglutide 3.0 mg | 2,487 | 1,789 | 45.2 | 106.2±21.2 | 38.3±6.4 | -8.0 |
| Placebo | 1,244 | 801 | 45 | 106.2±21.7 | 38.3±6.3 | -2.6 | ||
| Davies et al. [30] (2015)† | 56 | Liraglutide 3.0 mg | 423 | 310 | 55.0 | 105.7±21.9 | 37.1±6.5 | -6.0 |
| Liraglutide 1.8 mg | 211 | 154 | 54.9 | 105.7±21.9 | 37.0±6.9 | -4.7 | ||
| Placebo | 212 | 135 | 54.7 | 106.5±21.3 | 37.4±7.1 | -2.0 | ||
| Blackman et al. [31] (2016) | 32 | Liraglutide 3.0 mg | 180 | 134 | 48.6 | 116.5±23.0 | 38.9±6.4 | -5.7 |
| Placebo | 179 | 142 | 48.4 | 118.7±25.4 | 39.4±7.4 | -1.6 | ||
| O’Neil et al. [32] (2018) | 52 | Semaglutide 0.05 mg | 103 | 77 | 47 | 111.3±23.2 | 39.1±6.5 | -6.3 |
| Semaglutide 0.1 mg | 102 | 88 | 45 | 111.3±21.5 | 39.6±7.4 | -9.1 | ||
| Semaglutide 0.2 mg | 103 | 87 | 44 | 114.5±24.5 | 40.1±6.9 | -12.5 | ||
| Semaglutide 0.3 mg | 103 | 88 | 47 | 111.5±23.0 | 39.6±7.1 | -12.1 | ||
| Semaglutide 0.4 mg | 102 | 82 | 48 | 113.2±26.4 | 39.9±8.8 | -14.0 | ||
| Semaglutide 0.3 mg FE | 102 | 75 | 47 | 108.1±22.1 | 38.2±6.5 | -12.3 | ||
| Semaglutide 0.4 mg FE | 103 | 91 | 46 | 109.6±21.3 | 38.5±5.9 | -17.0 | ||
| Liraglutide 3.0 mg | 103 | 86 | 49 | 108.7±21.9 | 38.6±6.6 | -8.3 | ||
| Placebo | 136 | 103 | 46 | 114.2±25.4 | 40.1±7.2 | -2.3 |
Values are presented as number or mean±standard deviation. The mean weight loss was calculated for the full analysis set, which comprised all randomized individuals exposed to trial drug with at least one post-randomization weight assessment.
BMI, body mass index; FE, fast (2-weekly) dose escalation.
Table 4.
| Variable |
Author (year) |
|||||
|---|---|---|---|---|---|---|
| Bohula et al. [58] (2018)* | Nissen et al. [59] (2016)* | Marso et al. [60] (2016)† | ||||
| Noninferiority margin of HR | 1.4 | 1.4 | 1.3 | |||
| Follow-up duration | 3.3 y (3.0–3.5 y) | 121 wk (114−128 wk) with 2-week crossover run-in | 3.8 y with 2-week placebo run-in | |||
| Group | Lorcaserin 20 mg | Placebo | NB | Placebo | Liraglutide 1.8 mg | Placebo |
| No. | 6,000 | 6,000 | 4,455 | 4,450 | 4,668 | 4,672 |
| No. of AEs leading to discontinuation of study drug | 433 (7.22) | 220 (3.67) | 1,253 (28.1) | 388 (8.7) | 444 (9.5) | 339 (7.3) |
| Age (y) | 64 (58–69) | 64 (58–69) | 61.1±7.27 | 60.9±7.38 | 64.2±7.2 | 64.4±7.2 |
| Initial bodyweight (kg) | 102 (90–116) | 102 (90–116) | 105.6±19.1 | 106.3±19.2 | 91.9±21.2 | 91.6±20.8 |
| Initial body mass index (kg/m2) | 35 (32−39) | 35 (32−39) | 36.6 (33.1−40.8) | 36.7 (33.1−41.1) | 32.5±6.3 | 32.5±6.3 |
| Male | 3,888 (64.8) | 3,814 (63.6) | 2,018 (55.3) | 2,031 (55.6) | 3,011 (64.5) | 2,992 (64.0) |
| Baseline condition | ||||||
| Diabetes | 3,385 (56.4) | 3,431 (57.2) | 3,784 (84.9) | 3,803 (85.5) | 4,668 (100.0) | 4,672 (100.0) |
| Glycated hemoglobin (%) | 6.1 (5.7−7.0) | 6.1 (5.6−7.0) | 7.0 (6.1−8.1)‡ | 7.1 (6.4−8.2)‡ | 8.7±1.6 | 8.7±1.5 |
| Cardiovascular disease | 4,488 (74.8) | 4,470 (74.5) | 1,415 (31.8) | 1,447 (32.5) | 3,831 (82.1) | 3,767 (80.6) |
| Mean weight loss | -4.2 kg at 1 y | -1.4 kg at 1 y | -3.6% | -1.1% | Difference between group was 2.3 kg | |
| MACE at trial completion | 364 | 369 | 124 | 119 | 608 | 694 |
| MACE at interim analysis | 242 | 241 | 90 | 102 | NA | NA |
| All cause death | 212 | 202 | 43 | 51 | 381 | 447 |
| HR (95% CI) for MACE | 0.99 (0.85−1.14) | 0.95 (0.65−1.38)§ | 0.87 (0.78−0.97) | |||
Values are presented as number (%), median (interquartile range), or mean±standard deviation, unless otherwise stated.
HR, hazard ratio; NB, naltrexone 32 mg+bupropion 360 mg; AE, adverse event; MACE, major cardiovascular events (a composite of cardiovascular death, myocardial infarction, and stroke); NA, not applicable; CI, confidence interval.







