The Association between Obesity Phenotypes and Early Renal Function Decline in Adults without Hypertension, Dyslipidemia, and Diabetes
Article information
Abstract
Background
The prevalence of chronic kidney disease is increasing worldwide. Several studies have suggested that obesity is associated with early renal dysfunction. However, little is known about the relationship between obesity phenotypes and early renal function decline. Therefore, this study aimed to identify the relationship between obesity phenotypes and early renal function decline in adults without hypertension, dyslipidemia, and diabetes.
Methods
We conducted a cross-sectional analysis of clinical and anthropometric data from 1,219 patients who underwent a routine health checkup in 2014. We excluded adults with cardiovascular disease, renal disease, diabetes, hypertension, dyslipidemia, or low glomerular filtration rate (<60 mL/min/1.73 m2). Renal function was determined according to the estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C equation.
Results
Age, sex, body mass index, waist circumference, triglyceride, low-density lipoprotein, and fasting glucose had an association with the estimated glomerular filtration rate. After adjusting for age, sex, smoking status, and alcohol intake, the odds ratios of the metabolically abnormal normal weight and metabolically abnormal obese phenotypes for the presence of low estimated glomerular filtration rates were 1.807 (95% confidence interval, 1.009– 3.236) and 1.834 (95% confidence interval, 1.162–2.895), compared with the metabolically healthy normal weight phenotype. However, the metabolically healthy obese phenotype did not show a significant association with early renal function decline.
Conclusion
In this cross-sectional study, we confirmed the association between the metabolically abnormal normal weight and metabolically abnormal obese phenotypes and early kidney function decline in adults without hypertension, dyslipidemia, and diabetes.
INTRODUCTION
Obesity has been shown to be a risk factor for cardiovascular disease (CVD) and chronic kidney disease (CKD) [1-3]. The prevalence of CKD is increasing worldwide, and the economic burden of its treatment is increasing. Therefore, it is essential to prevent the progression of CKD by detecting renal dysfunction early [4,5].
In the longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study, higher body mass index (BMI) categories were associated with greater declines in kidney function in young adults with preserved glomerular filtration rate (GFR >90 mL/ min/1.73 m2 ) at baseline [6]. Previous studies have defined renal function decline as an estimated GFR (eGFR) of <60 mL/min/1.73 m2 , and included subjects with chronic illnesses such as hypertension (HTN) and diabetes. Furthermore, in a retrospective cohort study from Japan, the metabolically abnormal obese (MAO) phenotype, unlike the metabolically healthy obese (MHO) phenotype, was associated with higher risks of CKD [7]. However, recent studies reported that the MHO phenotype was associated with an increased incidence of CKD, indicating that obesity is a risk factor for CKD regardless of metabolic abnormalities [8-10].
Obesity is diagnosed on the basis of the BMI; however, BMI has a limitation in accurately describing the distribution of body fat in Asian people, who have a relatively higher proportion of body fat than other ethnic groups [11-13]. The phenotypes of obesity have recently been established and were classified into four groups according to the presence of metabolic syndrome and obesity based on BMI. This classification reflects body fat distribution more accurately than describing obesity based only on BMI or the presence of metabolic syndrome [14-17]. Although several studies have suggested that obesity is associated with early renal dysfunction [3,6], very few studies have focused on the association between early renal dysfunction and obesity phenotypes in adults without HTN, dyslipidemia, and diabetes.
The serum creatinine-based Modification of Diet in Renal Disease (MDRD) equation was used to measure GFR in most of the previous studies; however, its application to healthy individuals with GFR ≥60 mL/min/1.73 m2 is limited [18,19]. To overcome this limitation, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was introduced. Compared with CKD-EPI using only either creatinine or cystatin C, the CKD-EPI equation incorporating both creatinine and cystatin C (CKD-EPI creat-cys) has been shown to more accurately predict CKD progression [20].
Therefore, this study aimed to evaluate the association between early renal function decline and obesity phenotypes including metabolic components and BMI in adults without HTN, dyslipidemia, and diabetes, by using the CKD-EPI creat-cys equation.
METHODS
1. Subjects
We performed a cross-sectional study using the medical records of patients who visited the health assessment center at Pusan National University Yangsan Hospital, South Korea, for periodic health examinations between January 2014 and December 2014. Of 4,770 Korean subjects, we excluded the following from the study: those aged <35 years; those without eGFR and serum cystatin C measurements; those with HTN, diabetes, or dyslipidemia; those with CKD (diagnosed by a doctor or if the eGFR was <60 mL/min/1.73 m2 for 3 months before the examination); and those with CVDs (myocardial infarction, unstable angina, or stroke), malignant diseases, or severe hepatic diseases (liver cirrhosis, viral hepatitis, or hepatocellular carcinoma). Our total sample size was 1,219. The present study was approved by the institutional review board of Pusan National University Yangsan Hospital (IRB approval no., E-2018-068).
2. Data Collection and Measurements
Blood pressure was determined with the subjects in a sitting position after resting for approximately 10 minutes. Waist circumference (WC) was measured from the halfway point between the lower line of the last rib and the upper line of the iliac crest. BMI was calculated as weight in kilograms divided by height in meters squared.
All blood samples were collected in the morning after an overnight fast of at least 12 hours. Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels were measured with a Toshiba TBA200FR (Toshiba, Tokyo, Japan) using a direct measurement method, and triglyceride (TG) levels were measured using lipase, glycerol kinase, glycerol-3-phosphate oxidase, and peroxidase with a glycerol blank. Serum creatinine and cystatin C levels were estimated using the compensated Jaffe kinetic method (Beckman Coulter Inc., Fullerton, CA, USA) and latex-enhanced immunoturbidimetric assay (Diazyme Laboratories, Poway, CA, USA), respectively. Fasting glucose levels were measured using a glucose oxidase test method (LX-20; Beckman Coulter, Brea, CA, USA).
Smoking status was classified into non-smoking or current smoking. Alcohol consumption was classified as >1 drink per week or nondrinking.
The eGFR was calculated using the CKD-EPI creat-cys equation. A normal eGFR was defined as ≥90 mL/min/1.73 m2 , and early renal function decline was defined as an eGFR of 60–90 mL/min/1.73 m2 (Table 1).
3. Definition of Metabolic Abnormality
The diagnosis of metabolic syndrome was made according to the modified Adult Treatment Panel III, as proposed by the American Heart Association and National Heart, Lung, and Blood Institute in 2005 [21]. Abdominal obesity was defined as a WC of ≥90 cm in men and ≥85 cm in women, according to the Korean criteria of abdominal obesity [22]. Metabolic syndrome was diagnosed if at least three of the following criteria were satisfied: (1) WC ≥90 cm in men and ≥85 cm in women; (2) systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg, or antihypertensive medication use; (3) fasting plasma glucose (FPG) ≥100 mg/dL or antidiabetic medication use; (4) TG ≥150 mg/dL or antidyslipidemic medication use; and (5) HDL <40 mg/dL in men and <60 mg/dL in women, or antidyslipidemic medication use.
4. Definition of Obesity Phenotypes
According to the World Health Organization Asia Pacific guidelines, obesity was defined as a BMI of ≥25 kg/m2 . The obesity phenotype was divided into four types: metabolically healthy normal weight (MHNW), metabolically abnormal normal weight (MANW), MHO, or MAO. Subjects with MANW were defined as those without obesity but with metabolic abnormality. Obese subjects who had no other metabolic abnormalities were defined as having MHO. Subjects with MAO were defined as those with both obesity and metabolic abnormality.
5. Statistical Analysis
All statistical analyses were performed using the IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). For the comparison of continuous variables between the four groups, we performed one-way analysis of variance (ANOVA) with the post-hoc Scheffe or Games-Howell test. For cases assuming equal variance, one-way ANOVA was used. For cases not assuming equal variance, Welch’s ANOVA was used. For the comparison of continuous variables, we used the chi-square test. Pearson’s correlation analysis was used to determine the individual effects of age, BMI, WC, lipid, and FPG on eGFR. Subsequently, we performed multiple linear regression analysis to identify the independent determinants of eGFR. Moreover, a logistic regression analysis was used to determine the association between the obesity phenotypes and early kidney function decline. Assessments were performed after adjusting for age, sex, smoking, and drinking. P-values <0.05 were considered statistically significant.
RESULTS
The clinical characteristics according to metabolic phenotype in subjects with and without obesity are shown in Table 2. Of the 1,219 subjects, 66% (805/1,219) had MHNW, 5.6% (68/1,219) had MANW, 18% (220/1,219) had the MHO phenotype, and 10.3% (126/1,219) had the MAO phenotype. Men accounted for 51.2% (624/1,219) of the subjects. The mean age of the subjects was 52.69±8.57, and there was no difference in age according to sex. The baseline cystatin C level and eGFR showed significant differences in particular obesity phenotypes. The MHO group had significantly higher BMI, WC, SBP, DBP, TG, LDLcholesterol, FPG, and cystatin C values than the MHNW group (P<0.001, all). Similarly, almost all the variables (such as BMI, WC, SBP, DBP, TG, FPG, and cystatin C) were significantly different between the MHNW and MANW groups (P<0.001, all).
As shown in Table 3, Pearson’s correlation analysis revealed that the eGFR was negatively correlated with age (r=-0.396, P<0.001), BMI (r=- 0.221, P<0.001), WC (r=-0.392, P<0.001), SBP (r=-0.127, P<0.001), DBP (r=-0.188, P<0.001), TG (r=-0.192, P<0.001), LDL (r=-0.144, P<0.001), FPG (r=-0.157, P<0.001), and cystatin C (r=-0.764, P<0.001), and positively correlated with sex (r=0.517, P<0.001) and HDL (r=0.249, P<0.001).
We conducted a multiple logistic regression analysis to estimate the odds ratios (ORs) for early kidney function decline according to obesity phenotypes (Table 4). After adjusting for age, sex, smoking status, and alcohol intake, the ORs of the MANW and MAO phenotypes for the presence of low eGFR were 1.807 (95% confidence interval [CI], 1.009–3.236) and 1.834 (95% CI, 1.162–2.895), respectively. However, the MHO phenotype did not show a significant association with early renal function decline.
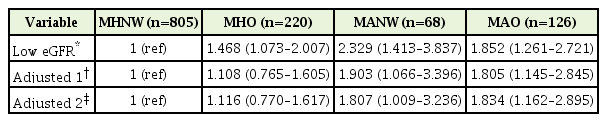
Association between early renal function decline and obesity phenotypes after adjusting for confounding factors
When stratified by sex, there was no significant association in the groups with early renal function decline compared with the MHNW group in men (n=624) (Table 5). However, in women (n=595), there was a significant OR increase in the MANW (OR, 3.782; 95% CI, 1.823– 7.847) and MAO (OR, 2.845; 95% CI, 1.338–6.046) groups compared with the MHNW group.
DISCUSSION
This study was designed to examine the association between early kidney function decline and obesity phenotypes in adults without HTN, dyslipidemia, and diabetes. In the present study, the MANW and MAO phenotypes were associated with significantly low eGFR and high cystatin levels, even after adjusting for age, sex, smoking status, and alcohol intake. However, there was no significant association between early renal function decline and the MHO phenotype.
Additional analysis according to sex found no significant association in the groups with early renal function decline compared with the MHNW group in men but not in women. The sex differences in our findings could be related to sex hormones. Male sex hormones induce negative effects within the kidney through various pathways such as the renin-angiotensin system, oxidative stress, and fibrosis [23]. In this study, it was difficult to confirm the effect of hormones because the male sex hormone levels were not measured. In addition, the small sample size might have also affected the different results between men and women.
The present study suggests that metabolic syndrome might contribute to the increasing risk of CKD. The possible mechanisms are not exactly known. Previous studies presumed that complex pathophysiological factors contributing to renal disease in patients with metabolic syndrome are mediated, such as insulin resistance, adipocytokines, endothelial dysfunction, renin-angiotensin-aldosterone-system activation, and oxidative stress [24,25]. However, recent studies reported that obesity is a risk factor for CKD regardless of metabolic abnormalities [8-10]. Our study can explain the difference in these results by investigating early renal function decline (eGFR 60–90 mL/min/1.73 m2 ) rather than chronic renal failure [26].
Yu et al. [27] suggested that obesity phenotypes do not significantly contribute to mildly reduced eGFR. Instead, sex, aging, dyslipidemia, and hyperglycemia were associated with an increased risk of mildly reduced eGFRs. They analyzed subjects with chronic diseases, not healthy adults. In addition, they used creatinine-based CKD-EPI to estimate GFR. On the other hand, our study analyzed healthy adult subjects without HTN, dyslipidemia, and diabetes, and our results showed that only obesity did not significantly correlate with early renal function decline and only had an impact on early renal function decline in the presence of metabolic syndrome.
Several studies have reported the existence of an association between early kidney function decline and obesity or metabolic syndrome. Grubbs et al. [6] found that higher BMI categories were associated with greater declines in kidney function in a cohort of young adults with preserved GFR (eGFR [based on cystatin C] >90 mL/min/1.73 m2 ) at baseline. De Boer et al. [28] also reported that obesity was associated with GFR decrease, calculated using the MDRD equation, in older adults.
In a retrospective observational cohort study conducted in Taiwan, metabolic components were positively associated with renal function deterioration, and treatment of metabolic syndrome was shown to attenuate CKD progression in patients with early-stage CKD [26]. Similarly, in a cross-sectional study from Spain, metabolic syndrome was significantly associated with a higher risk of early-stage kidney disease [29].
Many clinical laboratories use the serum creatinine-based MDRD equation to measure GFR. However, the MDRD equation systematically underestimates the GFR of relatively healthy individuals with GFR >60 mL/min/1.73 m2 [18,19]. The clinical efficacy of cystatin C has been recently confirmed, and previous studies have shown that the combined CKD-EPI creat-cys equation performs better than calculations using either creatinine or cystatin C alone, and may be useful as a confirmatory test for CKD [20,30]. In this study, the CKD-EPI creat-cys equation was also used to more accurately calculate the eGFR in healthy adults.
Our study has specific strengths. We selected healthy subjects without CVD, cancer, HTN medication use, diabetes mellitus, or dyslipidemia, and we used the new CKD-EPI equation to estimate the GFR, which has been proven to be more accurate and precise than the MDRD equation. However, this study also has several limitations. First, we could not include a sufficient number of subjects to provide good statistical power. Among the four groups, the MANW phenotype had the fewest number of subjects. The ratio of each of the four phenotypes was significantly different in men and women. Therefore, large-scale studies are needed in the future. Second, the duration of follow-up may have been insufficient to properly evaluate the risk of impaired renal function. Third, we could not control for the various potentially confounding factors associated with eGFR (e.g., smoking, alcohol, uric acid, and FPG). Fourth, the causal relationship among variables could not be determined with certainty owing to the cross-sectional nature of the study.
In this cross-sectional study, we confirmed the association between the MANW and MAO phenotypes and early kidney function decline in healthy adults. Further studies with a larger cohort of patients and with corrections for a variety of factors related to eGFR are required.
Notes
No potential conflict of interest relevant to this article was reported.




