Psoriasis and Cardiovascular Disease: A Narrative Review
Article information
Abstract
Psoriasis is a chronic, autoimmune, and inflammatory disease that affects 2% of the world’s population. In recent years, it has been demonstrated that psoriasis confers a 25% increase in relative risk of cardiovascular disease, independent of factors such as hyperlipidemia, smoking, and obesity. The objective of this review was to analyze and describe the association between psoriasis and cardiovascular disease. In this review, we describe the epidemiological association of psoriasis and cardiovascular disease, pathophysiology, mechanisms, and its association with the well-known cardiovascular risk calculators. In addition, we describe diagnostic tools, such as imaging techniques and novel biomarkers, that are useful in the evaluation of atherosclerotic cardiovascular disease. Finally, we present different systemic therapies that are used in patients with psoriasis and their effect on atherosclerotic cardiovascular disease. This article provides an overview of the current literature on psoriasis and cardiovascular risk, which can be useful for primary care physicians in their daily clinical practice.
INTRODUCTION
Psoriasis is a chronic, autoimmune, and inflammatory disease, with a prevalence of 0.09% to 5.1% of the world population and an incidence of 60.4 to 140 new cases per 100,000 [1,2]. Overall, the Caucasian population seems to be the most affected ethnicity. The disease affects all age groups; however, it presents a bimodal distribution, with a high incidence in the third and sixth decades of life [3]. Indeed, approximately 75% patients with this pathology develop symptoms before the age of 40 years [4].
Psoriasis confers a high relative risk of cardiovascular (CV) disease in 25% of patients, independent of factors such as hyperlipidemia, smoking, and obesity, and patients with severe psoriasis have an additional absolute risk of 6.2% of suffering a major adverse cardiovascular event (MACE) within 10 years of diagnosis in comparison with healthy populations [5,6]. The clinical approach to patients with psoriasis should include a comprehensive evaluation, in the context of CV risk stratification and management of CV risk factors [7].
CLASSIFICATION
Multiple classifications have been proposed in the evaluation of patients with psoriasis. An example includes bimodal age distribution: type I includes patients diagnosed before the age of 40 years and is associated with psoriasis in first-degree relatives, as well as more severe and recurrent behavior and; type II includes patients diagnosed after the age of 40 years [8]. According to the body surface area (BSA) affected, the disease can be classified as mild if the affected BSA is less than 2%, moderate if it is between 3% and 10%, and severe if the effect involves more than 10% of the BSA [9]. According to its morphological characteristics, psoriasis can be classified as plaque, pustular, guttata, and erythrodermic, with the first one being the most common and occurring in 90% of cases. Other categories are also described according to their distribution pattern, affected anatomic site, and stage of development [10].
EPIDEMIOLOGICAL ASSOCIATION BETWEEN PSORIASIS AND CARDIOVASCULAR DISEASES
The association between psoriasis and MACE has been described for decades. The first study was conducted in 1973, in which the authors observed a two-fold risk between psoriasis and arterial and venous disease [11]. Later, multiple studies were developed to evaluate the increased CV risk in patients with psoriasis; all of the studies described new diagnostic and therapeutic pathways in the management of patients with psoriasis [12].
In previous studies, Jung et al. [13] described a cohort of Korean patients with increased body mass index (BMI), total cholesterol, systolic blood pressure, prevalence of diabetes, obesity, and dyslipidemia at baseline than those of controls. In a longitudinal study, patients with psoriasis showed a higher hazard ratio (HR) for atherosclerotic CV disease (ASCVD) compared with controls after adjusting for several risk factors; moreover, both males and females stratified by sex also revealed a higher HR for patient with psoriasis, indicating that psoriasis is an independent risk factor for an increased incidence of ASCVD in this population. In this same population, males had a greater HR for ischemic heart diseases and angina pectoris than did females; in addition, HR is higher in patients with moderate and severe psoriasis than in patients with mild psoriasis when stratifying by disease severity. Also, ischemic stroke is more common in female than male patients. These authors conclude that psoriasis is related to a higher risk of ischemic CV events in both male and female Korean patients with severe psoriasis than in other patients [13].
Data regarding CV implications in elderly patients with psoriasis demonstrate that there is not a higher prevalence of this disease in patients older than 75 years with acute coronary syndrome (ACS) than in controls; this was recently demonstrated in a study by Morici et al. [14], in which Italian patients with ACS were compared with controls.
Another interesting implication of psoriasis that must be considered is the fact that it has a substantial impact on quality of life (QOL). Indeed, this disease is associated with an increased incidence of psychiatric illness (62%), with depression being the most prevalent. Recent data show that depression in patients with psoriasis may be linked to ischemic heart disease and cerebrovascular disease, making depression an independent risk factor for these diseases [15].
It has been shown that psoriasis increases mortality via both global and specific causes. In fact, psoriasis increases the relative risk of mortality from 1.12 to 1.52 compared to the general population. Regarding specific causes, CV disease showed an increase in the relative risk of mortality, ranging from 1.05 to 1.38. Meanwhile, the main non-CV causes of death in patients with psoriasis were liver and kidney diseases. Other causes that were also prevalent were infections, neoplasms, and chronic respiratory disease of the lower airways [16].
PATHOPHYSIOLOGY AND MECHANISM OF CARDIOVASCULAR DISEASE IN PSORIASIS
It is well known that psoriasis is an inflammatory disease in which Th1 lymphocytes activate antigen-presenting cells, Th1 cytokines, and T cells. When evaluating the cytokine profile in psoriasis and comparing it with that in vascular atherosclerotic lesions, an elevation of, both, Th1 and Th17 lymphocytes was observed. This overexpression of cytokines could induce atherogenesis and CV disease [17].
In psoriasis, lymphocytes release proinflammatory cytokines, such as interleukin (IL)-2 and interferon-α [18]. Other proinflammatory cytokines increased in psoriasis and linked to CV morbidity include IL-6, tumor necrosis factor (TNF), C-reactive protein (CRP), E-selectin, and intercellular adhesion molecule 1. Moreover, some cytokines could affect endothelial function and insulin sensitivity [19]. In psoriasis, Th17 cells and the expression of IL-17 are increased in skin lesions, related to a proatherogenic state [20,21].
It is important to identify the intermediate mechanisms present in the inflammatory process. Regulatory T cell functions are impaired in psoriasis, leading to a reduction in anti-inflammatory effects in atherosclerosis and psoriasis. Macrophages act as proinflammatory cells and play a role in atherosclerosis and vulnerability to plaque in psoriasis. Neutrophils also play a role in atherosclerosis and independently predict endothelial dysfunction, linking psoriasis and CV diseases [22].
Some risk factors are shared between psoriasis and CV risk and can exacerbate inflammation in diseases such as those for diabetes, hypertension, smoking, obesity, and dyslipidemia [3,23]. Inflammation is a prime factor in the development of atherosclerotic plaques and increases the risk of rupture resulting in the formation of a thrombus [24,25]. In inflammatory disorders, there are three characteristics that are commonly seen: accelerated coronary atherosclerosis, atrial fibrillation as a manifestation of atrial myopathy, and ventricular myopathy with a preserved ventricular ejection fraction. Systemic therapy targeting the reduction of adipose tissue mass and inflammation could be beneficial for both psoriasis and CV disease [26].
Vitamin D deficiency has been associated with multiple inflammatory and autoimmune diseases, such as type I diabetes, multiple sclerosis, inflammatory bowel disease, and rheumatoid arthritis and could be independent of traditional CV risk factors [27].
Reactive oxygen species oxidize lipids, proteins, low-density lipoprotein (LDL), and high-density lipoprotein (HDL), resulting in cell damage in patients with psoriasis. An increased number of cutaneous advanced glycation end products have been correlated with carotid intima-media thickness (CIMT) in patients with psoriasis and could be a factor in premature atherosclerosis [28-30].
Psoriasis is known to be an independent risk factor for CV disease; meanwhile age, BMI, metabolic syndrome, and smoking status have been found to increase the risk of psoriasis. Women with psoriasis have a risk of hypercholesterolemia and hypertension, and overweight women are more susceptible to type 2 diabetes than are women with normal weight [31,32]. In addition, as recently demonstrated by Fernández-Armenteros et al. [33], patients with psoriasis have a higher prevalence of traditional CV risk factors, such as type 2 diabetes, dyslipidemia, arterial hypertension, obesity, fasting basal glycemia, low HDL cholesterol, hypertriglyceridemia, and increased waist circumference than in patients without psoriasis. Additionally, the authors found a higher prevalence of ischemic heart disease and vascular-cerebral accidents in patients with psoriasis than in those without [33].
CARDIOVASCULAR RISK CALCULATORS IN PSORIASIS
The Framingham risk score (FRS) calculator estimates the 10-year absolute risk of having coronary artery disease [34]. Despite the usefulness of this tool, the calculator generally underestimates CV risk by evaluating risk factors individually; this occurs mainly in patients with chronic systemic inflammatory diseases, since patients with traditional risk factors constitute only a small portion of the total risk, and other factors are not included in the CV risk calculator [7,35]. In addition, a relationship between severity of the clinical manifestations of psoriasis and its high CV risk has not been found, which indicates that, regardless of the patient’s psoriasis area severity index (PASI), a comprehensive approach is needed for patients with psoriasis and CV disease [36].
Some studies have made comparisons to assess CV risk in patients with psoriasis. Reports have shown some variations in their results, with most agreeing that CV risk calculated in patients with psoriasis was comparable to that in the control group; however, in male patients, the FRS was higher than it was in women in both groups, as well as in adults over 60 years of age and patients with a prolonged disease duration. In addition, when stratifying patients according to their PASI, no significant difference was found between patients with increased severity of the disease compared to those who were minimally affected [37,38].
In inflammatory diseases, a taskforce provided by the European League Against Rheumatism suggested multiplication by a factor of 1.5 for CV risk algorithms in patients with inflammatory arthritis. When applied to different CV risk calculators commonly used worldwide, such as the Systematic Coronary Risk Evaluation, FRS, QRISK2 and Reynold’s Risk Score, the discriminative ability and calibration in patients with psoriatic arthritis is not improved [39].
IMAGING IN THE EVALUATION OF CARDIOVASCULAR DISEASE
Imaging is a useful tool to detect patients at risk of atherosclerosis without clinical symptoms via measurements of the CIMT and the identification of atherosclerotic plaque. One of the tools used is carotid ultrasound, which allows us to evaluate the presence of subclinical atherosclerosis. This tool helped identify subclinical atherosclerosis in patients with psoriasis, which occurs more frequently in patients with severe disease, as calculated by the PASI, with age being the independent risk factor that best predicts carotid intima media thickness [40]. Moreover, ultrasound can be used to detect subclinical atherosclerosis in femoral arteries. Gonzalez-Cantero et al. [41] demonstrated that femoral atherosclerotic plaque was more prevalent in patients with psoriasis than in those without, and that it was more prevalent than carotid plaque, thereby improving its detection in subclinical stages. These data make ultrasound a low-cost, high-performance technique that allows assessment of atherosclerotic damage in patients with psoriasis.
Coronary microvascular dysfunction (CMD) is characterized by reduced coronary flow reserve, which is not secondary to epicardial coronary artery stenosis, detected by transthoracic Doppler echography. Piaserico et al. recently stated that CMD is associated with severe psoriasis, psoriatic arthritis, hypertension, and disease duration greater than 6 years, suggesting that this technique is a reliable prognostic marker for patients with psoriasis without a history of CV events [42].
The perivascular fat attenuation index (FAI) is a recently developed technique that uses computed tomography (CT) to allow physicians to evaluate coronary inflammation using differential mapping of attenuation gradients in pericoronary fat. Elnabawi et al. [43] demonstrated a reduction in the FAI of patients receiving biologic therapy for moderate to severe psoriasis, making this a novel tool to evaluate the effects of psoriasis therapy for coronary artery disease.
Echocardiography is a widely used imaging tool for evaluating cardiac function. In patients with psoriasis, left ventricular diastolic dysfunction was found in 36.5% of patients and was associated with an increase in mitral regurgitation compared to a control group in which none of the patients presented this alteration [44].
Another imaging technique that provides support in CV evaluations of patients is the level of coronary arterial calcium (CAC). CAC is a marker of subclinical atherosclerosis associated with CV events. This marker is associated with coronary artery disease in patients with severe psoriasis and is associated with high CAC values, making this technique an innovative tool for the evaluation of asymptomatic CV disease [45].
Few studies have compared both techniques for the evaluation of CV risk in psoriasis. By extrapolating these two techniques in other inflammatory diseases, carotid ultrasound has been found to have a higher sensitivity than CAC has [46].
Positron emission tomography with CT using F18-fluorodeoxyglucose (FDG-PET/CT) is used for benign inflammatory diseases due to the increase in glycolytic activity associated with these conditions, as well as for visualizing the arterial inflammation that occurs in these diseases. In patients with psoriasis, an increase in arterial, hepatic, and psoriatic lesions has been demonstrated by using the FDG-PET/CT technique. In addition, in patients diagnosed with moderate or severe disease, there is an increase in arterial and hepatic inflammation, making this a tool that can be used for the detection of subclinical atherosclerosis [47-49].
NOVEL BIOMARKERS
Biomarkers are useful in the evaluation of systemic inflammation in psoriasis. N-terminal pro B-type natriuretic peptide (NT-proBNP) is an inactive hormone that is derived secondary to the breakdown of BNP, which is produced by cardiomyocytes secondary to increased wall tension. NT-proBNP has been used to evaluate CV disease; indeed, in patients with psoriasis, this inactive hormone is increased compared to healthy controls, due to the high risk of CV disease in affected patients [50].
Homocysteine is an amino acid that has been widely used in inflammatory diseases. Patients with severe psoriasis have higher levels of homocysteine than those in patients with mild psoriasis, which correlates with the BSA affected but not with the psoriasis activity score index [51].
Galectin-3 (Gal-3) is a protein that is produced by macrophages, eosinophils, and epithelial cells. Proinflammatory processes, including psoriasis, increase Gal-3 levels; moreover, in skin lesions, Gal-3 is absent in the epidermis. Patients with psoriasis have higher levels of Gal3 than in healthy controls, leading to enhanced profibrotic activity [52].
IMPLICATIONS OF SYSTEMIC TREATMENT IN CARDIOVASCULAR DISEASE
For many years, there was a theory that anti-inflammatory treatment in psoriasis decreased the risk of MACE [53]. Subsequently, multiple studies evaluating the efficacy of systemic therapy in reducing CV risk have been developed. Table 1 shows different systemic therapies, their mechanism of action, implications in CV effect, and dermatological findings [15,21,54-94].
Methotrexate is a drug used as a first-line treatment in psoriasis [54]. This drug is one of the most accessible systemic therapies in terms of cost and could be used by primary care physicians (PCP). Patients undergoing treatment with methotrexate have a lower risk of CV disease than in other patients, and this reduction is greater in patients with low cumulative doses of the treatment, with a relative risk of 0.73 and a HR of 0.42. Additionally, the added use of folic acid, which prevents anemia in patients with methotrexate, contributes to the reduction of this risk, specifically cerebrovascular disease [55-58]. Previous studies have been performed in patients with rheumatoid arthritis and concomitant plaque psoriasis, in which the carotid-intima media thickness and femoral intima-media thickness were reduced in patients undergoing this disease-modifying anti-rheumatic drug (DMARD) therapy with 20 mg per week of methotrexate compared to those in patients prescribed with lower doses or different DMARDs, such as cyclosporine or biologics [59].
When patients do not respond to topical therapies or methotrexate treatment, they should be referred to a dermatologist. There are several other therapies that could be used for patients with psoriasis.
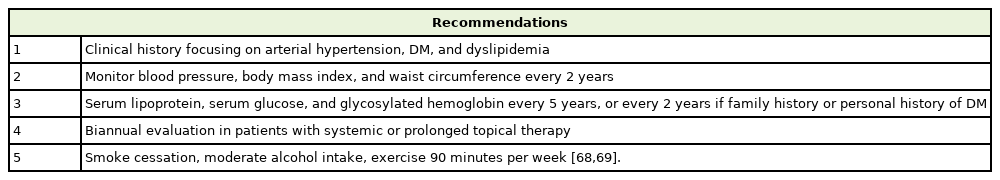
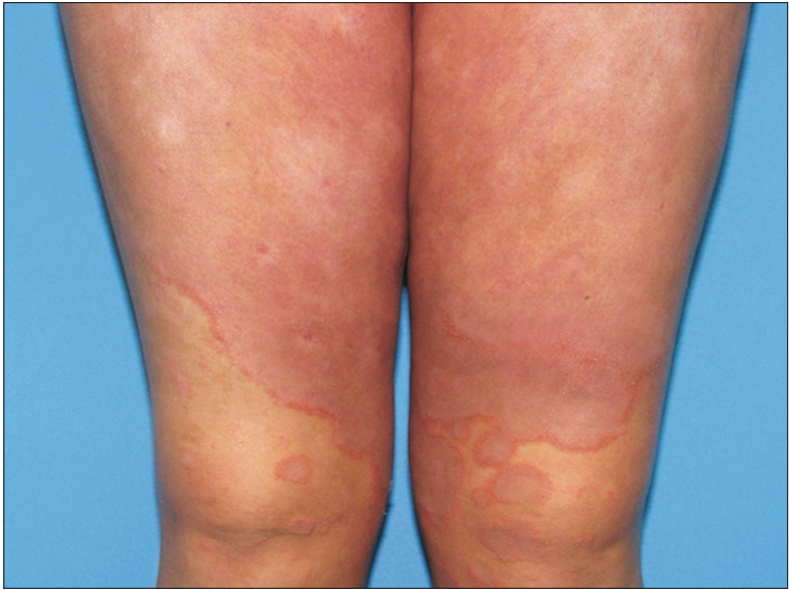
Cyclosporin is a common drug used as treatment for moderate to severe psoriasis [60,61]. Due to the widely known implications and side effects that cyclosporine has, treatment with this drug should be monitored by a dermatologist. Treatment of psoriasis with cyclosporine has some adverse effects that may increase CV risk in patients; in addition, patients with a high BMI are less likely to show improvement when using this drug [62,63]. It is recommended that cyclosporin should be used for a short amount of time, maximum a year, and a change to other systemic therapies should be considered when skin lesions have healed [15]. Figures 1 and 2 illustrate a patient using cyclosporin before and after treatment.
Patient with erythrodermic psoriasis, with psoriasis area severity index of 23.5. Written informed consent for publication of this image was obtained from the patient.
Patient with erythrodermic psoriasis, 10 weeks after initiation of cyclosporin treatment. Psoriasis area severity index of 1.3. Written informed consent for publication of this image was obtained from the patient.
Retinoids increase triglycerides and cholesterol levels, and change HDL cholesterol to LDL cholesterol. Acitretin is associated with a decrease in resistin levels and dose-dependent elevation of cholesterol and triglyceride serum parameters; however, this effect could be altered with diet and changes in dosage [64]. On the other hand, use of etretinate was associated with high cholesterol and triglyceride levels, as previously stated; however, this increase is not associated with a greater CV risk with prolonged use of this drug [65].
Colchicine improves psoriasis lesions and is beneficial as a maintenance therapy after lesions have healed [66]. This drug has been studied for its effects in CV diseases, showing that low doses prevent CV events in patients with stable coronary disease [95,96].
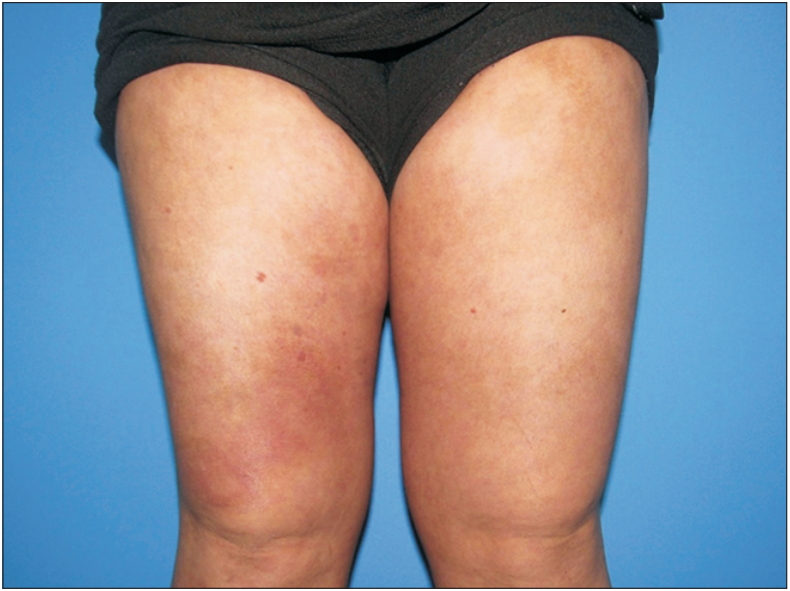
A new treatment model for psoriasis is biological therapy, which is classified into three main groups: T lymphocyte modulating agents, such as alefacept and efalizumab; inhibitors of TNF-α, including adalimumab, infliximab, and etanercept; and inhibitors of the IL-12 and IL-23 pathways, such as ustekinumab and briakinumab [97]. In addition to using the previously mentioned pathways, treatment was added that involves the IL-17 pathway, such as secukinumab, ixekizumab, and brodalumab or guselkumab, which is against IL-23 [98]. Figures 3 and 4 illustrate a patient using secukinumab before and after treatment.
Patient with plaque psoriasis, with psoriasis area severity index of 12.8. Written informed consent for publication of this image was obtained from the patient.
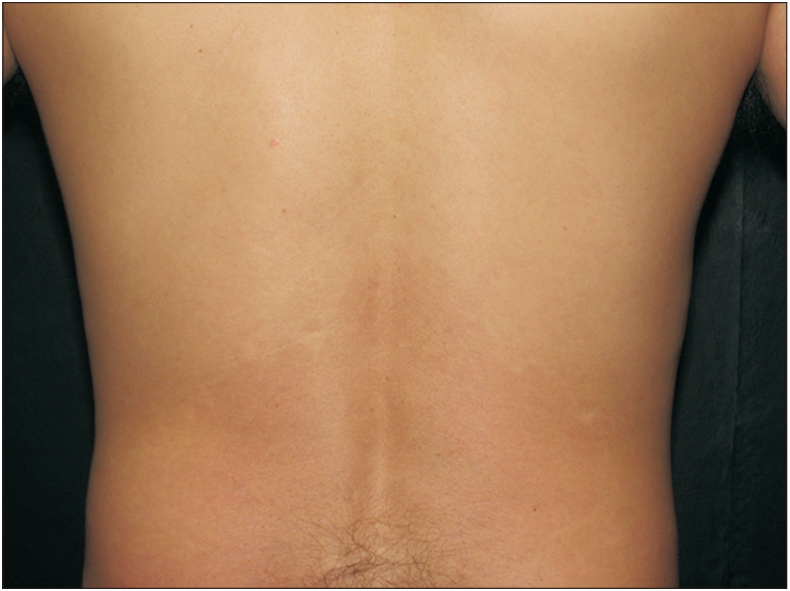
Patient with plaque psoriasis, 8 weeks after initiation of secukinumab treatment. Psoriasis area severity index of 0.4. Written informed consent for publication of this image was obtained from the patient.
Although some studies indicate that the use of TNF inhibitors, anti-IL-12/23, and anti-IL17A have no impact on the risk of MACE, it has recently been shown that biological therapy reduces the growth of non-calcified plaque, modifying the morphology of coronary plaque; therefore, it is necessary to evaluate each of these groups in detail to improve evaluation, as well as clinical and therapeutic decisions [99].
Inhibitors of TNF-α reduce CRP, vascular endothelial growth factor, and Th17; in addition, they block the differentiation of CD4+ T lymphocytes to Th1, Th17, and Th22, and the subsequent release of IL-17A, IL-17F, and IL-22 [20]. Adalimumab is contraindicated in patients with moderate to severe heart failure; however, inhibitors of TNF-α improve endothelial function by reducing the risk of myocardial infarction [21,67]. One year of treatment with this drug has also been shown to decrease the levels of IL-6 and TNF, and the acetylation of glycoproteins [69]. Retinol binding protein 4 is a retinol transporter protein that is linked to subclinical atherosclerosis, as it is correlated with the CIMT, and a reduction in the level of this protein occurs during treatment with TNF inhibitors [70,71,100]. On the other hand, etanercept improves vascular function by reducing CRP and improving insulin resistance [71,72].
Among the inhibitors of IL-12 and IL-23, briakinumab showed no significant difference in MACE compared to a placebo group. With respect to ustekinumab, a 5-year follow-up of more than 12,000 patients with psoriasis did not reveal any CV disease. Additionally, guselkumab does not appear to alter the metabolism of glucose and lipids [73].
IL-12 and IL-23 have been implicated as potential factors of atherogenesis, since atherosclerotic plaques contain monocytes producing IL-12, as well as increased cytokines induced by IL-23 [20,74]. Many of the patients who are recruited in these studies have a high number of CV risk factors, such as diabetes mellitus, hypertension, hyperlipidemia, obesity, and smoking, which must be considered when deciding which therapy to use. On the other hand, treatment with ustekinumab decreases systemic inflammation [75]. Other studies have shown that ustekinumab increases CV risk of MACE in patients with psoriasis. However, overall analysis suggests that this biological agent neither beneficially nor harmfully affects CV events [20]. With respect to briakinumab, the appearance of multiple deaths associated with MACE have been reported in a clinical trial, with an increased risk of such events in patients with two or more CV risk factors [76]. However, another study did not show a statistically significant difference when comparing these events with those of a placebo group. For these reasons, definite conclusions could not be made concerning briakinumab and the high risk of MACE events.
The effect of treatment with inhibitors of IL-17A on vascular inflammation is still unclear; however, analyses of patients using secukinumab support the idea that this inflammatory treatment decreases the CV effect in patients with moderate to severe psoriasis treated with a 300 mg dose when low rates of MACE are found [77,78]. Ixekizumab has not been demonstrated to have a substantial effect on reducing parameters with a CV impact [79]. On the other hand, brodalumab has not been shown to cause major CV events compared to ustekinumab in patients who have low levels of MACE, i.e., less than 0.4 events per 100 patientyears [80,81].
Canakinumab has been approved for use in autoinflammatory diseases, such as rheumatoid arthritis, periodic syndrome associated with cryopyrin, and multisystem inflammatory disease of neonatal onset, and has also been reported to be effective in diseases such as pustular psoriasis. Patients who have used canakinumab have achieved lower concentrations of CRP, leading to a significant reduction in MACE, CV mortality, and any other cause of death, despite having no effect on HDL cholesterol [82,83]. In addition, Ridker et al. [101] demonstrated these same effects when using a subcutaneous 150 mg posology every 3 months; however, another study conducted an evaluation of the area of the carotid wall and the aortic distensibility, and no difference was found compared to a placebo group [101,102].
ROLE OF THE PRIMARY CARE PHYSICIAN: MANAGEMENT, CONTROL, AND PREVENTION OF CARDIOVASCULAR DISEASE
Table 2 shows a series of recommendations that PCP should follow for the comprehensive management, control, and prevention of CV disease in patients with psoriasis [68,69]. A complete clinical history should be developed with a focus on comorbidities, such as hypertension, diabetes mellitus, and dyslipidemia. Blood pressure, BMI, and waist circumference should be reevaluated at every 2 years. It is also recommended that serum lipoprotein, glucose, and glycosylated hemoglobin tests be performed at every 5 years, or every 2 years if the patient has a positive hereditary family history or diabetes mellitus. Some guidelines even recommend a biannual evaluation in patients with systemic therapy or prolonged use of topical treatments. Additional recommendations include smoking cessation, moderation of alcohol intake, and 90 minutes of exercise per week [103,104].
CV risk should be evaluated and managed in patients with psoriasis. Recent studies suggest that hypertension and dyslipidemia are underdiagnosed and undertreated, mainly due to a combination of suboptimal adherence to treatment recommendations and inadequate treatment. Other factors that have been proposed as possible explanations of these gaps between suboptimal diagnosis and inadequate treatment are unawareness of CV disease, treatment focused on active disease, unknown CV prevention possibilities, and CV disease therapeutic guidelines [105]. In the evaluation and management of patients with psoriasis, stratifying CV risk could contribute to the prevention of CV morbidity and mortality, and could guide the PCP and the dermatologist to choose the best therapy for each patient.
PCP are cornerstones in the prevention and control of CV disease in psoriasis. Increasing the awareness of patients and health workers is an important step in improving patients’ CV risk. Semb and Rollefstad [106] suggested that official programs for educating patients and health workers should be implemented to lower CV risk in patients with psoriasis and psoriatic arthritis. PCP in their outpatient clinics, along with non-physician health workers, must make a joint effort to enhance patient communication, thereby contributing to CV disease control and prevention [106].
Multiple studies have shown an association between psoriasis and nutritional effects on CV risk. The Mediterranean diet is a source of antioxidants and monounsaturated fatty acid compounds with antiinflammatory properties and is associated with a lower CV risk. Indeed, PASI, BSA, and physician global assessment levels are lower in patients that adhere to this dietary regimen than in other patients; moreover, patients with severe psoriasis have a low adherence to a Mediterranean diet [107,108].
CONCLUSION
Psoriasis is a chronic and inflammatory disease associated with multiorgan and CV disease. An integrated evaluation and multidisciplinary approach are necessary to increase the QOL and life expectancy of patients with psoriasis. The role of the PCP is to choose the best treatment for decreasing, both, skin lesions and atherosclerotic damage caused by this condition, and referring the patient to the dermatologist when they do not respond to first-line treatment. Also, the PCP must educate the patients and their work team to enhance the communication between the patient and the physician.
Notes
No potential conflict of interest relevant to this article was reported.