Exposure to Secondhand Smoke and a Tobacco-Specific Carcinogen in Non-Smokers
Article information
Abstract
Background
The International Agency for Research on Cancer classifies 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) as a known carcinogen. This study aimed to investigate the association between exposure to secondhand smoke (SHS) and NNAL concentrations in non-smokers.
Methods
This was a cross-sectional study based on data from the 2016 to 2018 Korea National Health and Nutrition Examination Survey. Urine NNAL concentrations were categorized into tertiles of 3,615 study participants who were non-smokers. All sampling and weight variables were stratified, and analyses to account for the complex sampling design were conducted.
Results
The overall, male, and female percentages of SHS exposure among non-smokers were 22.4%, 29.2%, and 20.4%, respectively. The geometric means of urine NNAL concentrations were 1.896±0.098 pg/mL and 1.094±0.028 pg/mL in the SHS exposure and non-exposure groups, respectively. After adjusting for confounding variables, in the total group, the geometric mean of urine NNAL concentrations was significantly higher in the SHS exposure group than in the SHS non-exposure group (adjusted P-value <0.001). Compared with the non-exposure group, the adjusted odds ratios (95% confidence intervals) for the highest NNAL tertile group of overall SHS exposure in the total, men, and women groups were 2.44 (1.95–3.05), 1.65 (1.08–2.53), and 2.73 (2.11–3.52), respectively, after full adjustment.
Conclusion
The urine NNAL concentration in the SHS exposure group was significantly higher than that in the non-exposure group. Exposure to SHS was associated with a higher risk of elevated urine NNAL concentrations in non-smokers.
INTRODUCTION
The annual cancer statistical report of the Korea Central Cancer Registry reported age-standardized lung cancer mortality rates of 29.4 for men and 7.4 for women per 100,000 in 2017, and estimated that lung cancer is the leading cause of death in both sexes [1]. In Korea, the smoking rate among adult male smokers has steadily declined from 47.8% in 2008 to 35.7% in 2019, but the smoking rate among adult female smokers remains almost unchanged [2]. Despite the decrease in the smoking rate, the number of non-smokers who develop lung cancer is increasing [3,4], especially in South Korea, where the proportion of lung cancer adenocarcinoma histologic types is rising [5]. N-nitrosamines in tobacco smoke induce lung cancer adenocarcinomas that are common to non-smokers and individuals exposed to secondhand smoke (SHS) [3].
The World Health Organization Tobacco Free Initiative describes SHS as “the smoke emitted from the burning end of a cigarette or from other tobacco products usually in combination with the smoke exhaled by the smoker.” [6] The disease burden due to SHS was estimated to be 0.9 million deaths and 24 million disability-adjusted life years lost globally in 2015 [7]. SHS is associated with various diseases in addition to lung cancer [8-10]. A meta-analysis reported that SHS exposure at the work place increased the risk of lung cancer among non-smokers [11]. Thus, the International Agency for Research on Cancer (IARC) and US Environmental Protection have classified SHS as a carcinogen [12].
The IARC reported that 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a group 1 human carcinogen [13]. NNK and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is carcinogenic in animal models, and plays an important role in the pathogenesis of lung cancer in tobacco smokers [14,15]. NNAL is a reliable marker for detecting exposure to tobacco smoke and estimating the amount of NNK in the body [16,17]. However, few studies have investigated urine NNAL concentrations in SHS-exposed groups in Korea.
The purpose of this study was to investigate the association between SHS exposure and urine NNAL concentrations in non-smokers using the Korea National Health and Nutritional Survey (KNHANES).
METHODS
1. Data Source
The KNHANES is a cross-sectional and nationally representative survey conducted by the Korea Disease Control and Prevention Agency (KDCA; formerly Korea Centers for Disease Control and Prevention) according to the National Health Promotion Act. The KDCA provided more detailed information about the KNHANES in previous studies [18]. To maintain the representativeness of the Korean population, the KNHANES used a complex and multi-step probability sample design for applying sampling weights. The KNHANES also includes information about health status, health behavior, socioeconomic demographics, and laboratory tests. These data were obtained by trained interviewers during face-to-face interviews with participants and through the evaluation of laboratory test results. This study was approved by the Institutional Review Board of Chungbuk National University Hospital (CBNUH-2020-09-012) and followed the guidelines of the Declaration of Helsinki (1975). The Ethics Committee waived the necessity for informed consent because data were anonymized at all stages, including during data clearing and statistical analysis.
2. Study Population and Secondhand Smoke Exposure
This study was conducted among adult non-smokers aged over 19 years using data from the 2016 to 2018 KNHANES. Non-smokers were operationally defined as those who answered “no” to the question “How many cigarettes have you smoke in your lifetime?” Of the 11,025 eligible participants, individuals with missing urine NNAL concentration values (n=7,280), values below the detection limit (n=28), who previously smoked electronic cigarettes (n=1), and/or with urinary cotinine levels above 50 ng/mL [19] (n=101) were excluded. A total of 3,615 participants were included in the final analyses, with 803 and 2,812 in the SHS exposure and non-exposure groups, respectively (Figure 1).
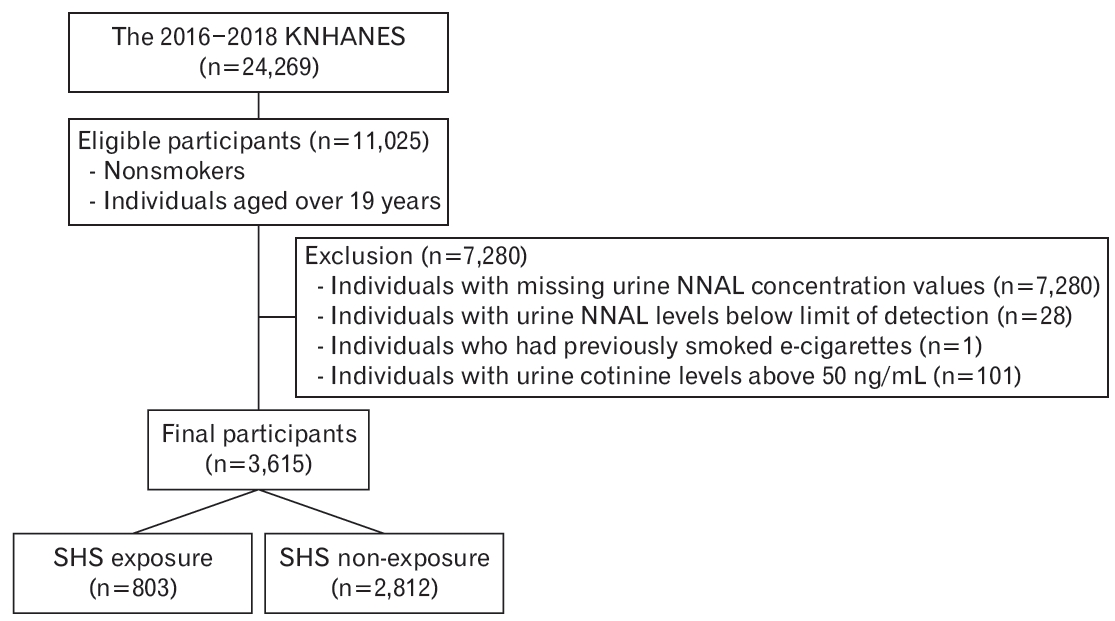
Flowchart of inclusion and exclusion. KNHANES, Korea National Health and Nutritional Survey; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; SHS, secondhand smoke.
The questionnaires were composed of three questions: “Have you experienced SHS at workplaces, at home, or in public places within the last 7 days?” If a participant answered “yes” they were defined as part of the SHS exposure group. According to the place of exposure, we further classified the participants into workplaces, homes, and public places.
3. Measurements of NNAL, Blood Pressure, and Body Mass Index
Urine NNAL concentration was measured by HPLC-MS/MS using an Agilent 1200 Series instrument with a Triple Quadrupole 5500 detector (AB Sciex, Framingham, MA, USA). Internal and external quality control teams validated the test results of the KNHANES laboratory analyses. These validation results met the criteria of the German Extend Quality Assessment Scheme for Analyses in Biological Materials, Centers for Disease Control and Prevention Ensuring the Quality of Urinary Iodine Procedures, and the College of American Pathologists survey. The detection limit of the urine NNAL concentration was 0.1006 pg/mL. Urine NNAL data were collected from the 2016 to 2018 KNHANES. Blood pressure was measured by a trained investigator, who used a standard mercury sphygmomanometer (Baumanometer; Baum Co. Inc., Copiague, NY, USA). The participants wore light indoor clothes and no shoes, and trained staff measured their body weight and height to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI, kg/m2) was calculated as weight (kg) divided by height squared (m2).
4. Definition of Variables
Health-related lifestyle information was obtained from data gathered using a self-reported questionnaire. The participants were divided into three groups according to urine NNAL concentration in the total, male, and female cohorts, respectively: T1, <0.786, <0.868, and <0.756 pg/mL; T2, 0.786–1.669, 0.868–1.900, and 0.756–1.564 pg/mL; and T3, >1.669, >1.900, and >1.564 pg/mL. Monthly average household income was calculated by dividing the total monthly household income by the square root of the number of family members. Sufficient physical activity was defined as individuals who engaged in moderate-intensity physical activity for ≥150 minutes per week or vigorous-intensity physical activity for ≥75 minutes per week. Men and women who drank at least seven and five cups of alcohol per day, respectively, and more than twice a week were classified into the heavy alcohol intake category. Occupational status was stratified into manual workers (clerks; service and sales workers; skilled agricultural, forestry, and fishery workers; persons who operate or assemble crafts, equipment, or machines; and elementary workers), office workers (general managers, government administrators, professionals, and simple office workers), and other (unemployed persons, housekeepers, and students). Educational status was divided into four groups according to education duration: less than elementary school graduation (<6 years), middle school (6–9 years), high school (9–12 years), and college or more (≥12 years). Marital status was classified into three groups: married and not separated, single (those who are not married, married but are now separated, married but whose spouses passed away, and divorced), and non-responders.
5. Statistical Analyses
Continuous and categorical variables were presented as mean±standard error (SE). All the sampling and weight variables were stratified. The SAS survey was adopted for statistical analysis to account for the complex sampling design and maintain national representativeness. Adjusted P-values were calculated by analysis of covariance with weighting of survey design, using log-transformed urine NNAL level (adjusted for age, sex [only in total group], BMI, SBP, income status, marital status, education duration, occupation, physical activity, and alcohol drinking). In addition, multivariate logistic regression analyses were performed to calculate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) associated with the high NNAL group after stratification according to sex and place of SHS exposure. Statistical analyses were conducted using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at a two-tailed P-value of <0.05.
RESULTS
Table 1 describes the characteristics of non-smokers according to SHS exposure. The unweighted numbers of SHS exposure and non-exposure were 803 and 2,812, respectively. Individuals exposed to SHS were younger than those who were not (42.9 versus 48.4 years), and the non-exposure group included more women. The non-exposure group also included a higher proportion of individuals who were married and not separated, less educated, less engaged in physical activity, and less likely to drink heavy alcohol than those of the exposure group. Supplement 1 presents the characteristics of non-smokers based on SHS exposure according to sex. Men who were not exposed to SHS were likely to be less or more educated (<6 years and ≥12 years), office workers, and less engaged in sufficient physical activity, than those exposed to SHS. Women not exposed to SHS were more likely to be older, married and not separated, less educated, less employed, less engaged in sufficient physical activity, and unlikely to drink alcohol heavily, than those exposed to SHS.
Figure 2 presents the proportion of individuals exposed to SHS according to sex. The percentages of overall SHS exposure in men and women were 22.4%, 29.2%, and 20.4%, respectively. Men were more likely to be exposed to SHS than women with respect to overall exposure (29.2% versus 20.4%), workplace exposure (12.4% versus 5.2%), and exposure in public places (22.2% versus 13.7%) (all P<0.001). However, the percentage of exposure to SHS at home was significantly higher for women (men, 3.5%; women, 6.4%; P=0.010).
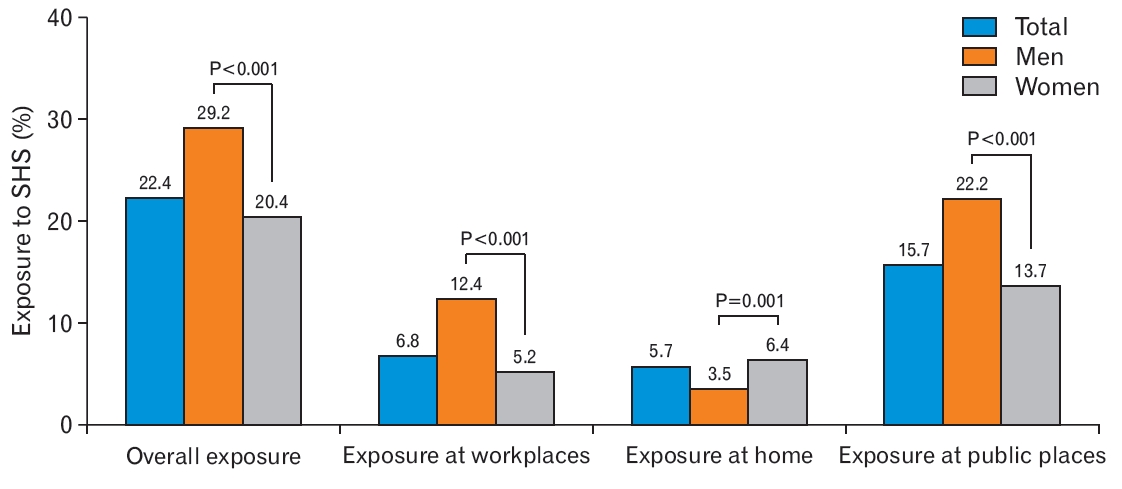
Proportion of exposure to secondhand smoke (SHS) according to sex. The P-values were calculated by chi-square test.
Table 2 presents the estimated mean, geometric mean, interquartile range, and median urine NNAL levels according to SHS exposure. The geometric means of urine NNAL concentrations were 1.896±0.098, 1.904±0.175, and 1.893±0.118 pg/mL for the total study population, male participants, and female participants, respectively. Urine NNAL concentrations in the non-exposed group were 1.094±0.028, 1.281±0.067, and 1.049±0.02 pg/mL for the total study population, male participants, and female participants, respectively (Figure 3). After adjusting for age, sex, BMI, SBP, marital status, income status, education attainment level, occupation, physical activity, and alcohol consumption in the total group, the urine NNAL concentrations were significantly higher in the SHS exposure group than in the non-exposure group (adjusted P<0.001) (Figure 3).
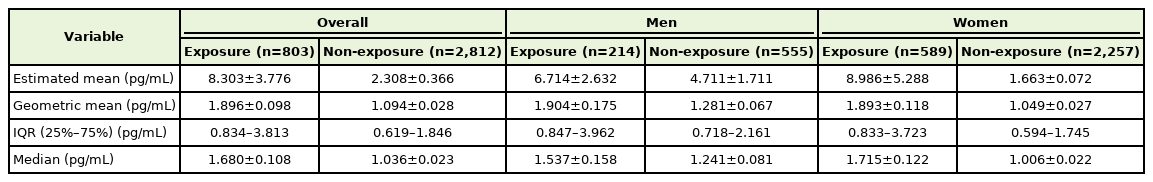
Estimated mean, geometric mean, IQR, and median of urine NNAL level according to secondhand smoke exposure

Blood geometric mean concentration of urine NNAL according to secondhand smoke exposure by sex. Adjusted P-values were calculated by analysis of covariance with weighting of survey design, using log-transformed urine NNAL level adjusted for age, sex (only in the total group), body mass index, systolic blood pressure, income status, marital status, education duration, occupation, physical activity, and alcohol consumption. NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
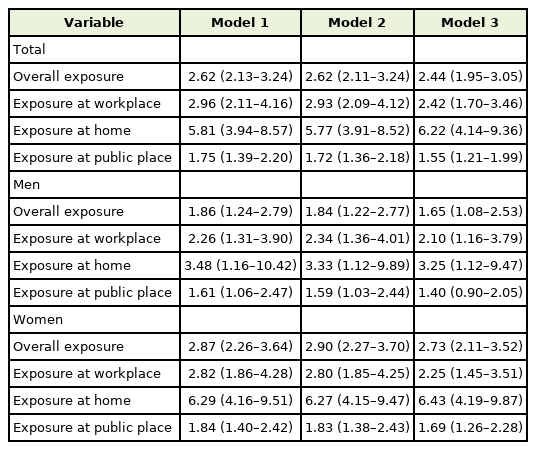
Table 3 shows the multivariate logistic regression analysis for T3 of the highest NNAL concentrations, according to the place of SHS exposure by sex. Compared with the non-exposure group, the adjusted ORs (95% CIs) for T3 of overall exposure, exposure at workplace, home, and public places were 2.44 (1.95–3.05), 2.42 (1.70–3.46), 6.22 (4.14–9.36), and 1.55 (1.21–1.99) in the total group, respectively (model 3), after adjusting for age, sex, BMI, SBP, income status, marital status, education duration, occupation, physical activity, and alcohol drinking. In men, adjusted ORs (95% CIs) for T3 of overall exposure, exposure at workplace, home, and public places were 1.65 (1.08–2.53), 2.10 (1.16–3.79), 3.25 (1.12–9.47), and 1.40 (0.90–2.05), respectively, after full adjustment. In women, all adjusted ORs for T3 of SHS exposure, regardless of place, were statistically significant: overall exposure (OR, 2.73; 95% CI, 2.11–3.52), exposure at workplace (OR, 2.25; 95% CI, 1.45–3.51), home (OR, 6.43; 95% CI, 4.19–9.87), and public places (OR, 1.67; 95% CI, 1.26–2.28), even after full adjustment (model 3).
DISCUSSION
This study showed that the urine NNAL concentration in the SHS exposure group was significantly higher than that in the non-exposure group, after adjusting for confounding variables using national representative data. After stratifying according to the SHS exposure place, the adjusted ORs for both men and women were statistically significant regardless of exposure places, compared to those in the non-exposure group, except for male exposure in public places.
Although the male smoking rate has been declining in Korea [2,20], the incidence of lung adenocarcinoma has been steadily increasing in both women and men and is replacing squamous cell carcinoma as the most common type of lung cancer in Korea [3]. The proportion of lung cancer adenocarcinoma histological types in non-smokers is also increasing [4,5]. This trend change in smoking rate and the proportion of lung cancer is leading to an increased social interest in SHS exposure. A Korean study reported that SHS exposure tended to consistently increase between 2008 and 2011 in non-smokers [21].
SHS is known to be associated with various diseases, in addition to lung cancer [8-10,22]. According to large meta-analysis studies, SHS may increase the overall risk of cancer, particularly lung cancer, for non-smokers, especially in women [11,21,23]. According to recent reports, SHS exposure has also been reported to be significantly associated with hypertension in female non-smokers [22]. The carcinogens contained in tobacco smoke are known to include polycyclic aromatic hydrocarbons (PAH), hetero-cycles, heterocyclic aromatic amines, tobacco specific N-nitrosamines, aldehydes, cadmium, and other miscellaneous organic compounds [24,25]. Of these carcinogens in tobacco smoke, tobacco specific N-nitrosamines and PAH are the most closely associated with lung cancer [26]. The N-nitrosamines in tobacco smoke include N-nitrosodimethylamine and N-nitrosopyrrolidine, as well as tobacco specific nitrosamines, such as N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) [27]. In humans, NNK is converted to its urinary metabolites, NNAL, and provides a biomarker of carcinogen exposure. The large amount of carcinogens in tobacco smoke inhaled by smokers is transferred to individuals exposed to SHS, even though there is a slight difference in compounds between active tobacco smoke and SHS [28].
In the lungs of individuals exposed to SHS, carcinogenesis may be similar to that of active smokers, despite that the amount of carcinogens differ between the two groups (active smokers inhale higher doses of carcinogens from tobacco smoke) [25]. Carcinogens such as N-nitrosamines are transformed into metabolites for detoxification and excretion from the body [29]. During this metabolic detoxification process, carcinogens are unintentionally converted into more toxic forms that react with DNA and cellular macromolecules, and this results in the formation of nucleic acid and protein adducts [29].
NNK is present in all tobacco products and is tobacco-specific and is considered a strong carcinogen [30]. NNAL is a metabolite of NNK and provides a specific biomarker for tobacco exposure. Positive NNAL urine test results indicate that the individual was exposed to potent tobacco-specific lung carcinogens and may potentially experience lung cancer development [31]. In the present study, after adjusting for several variables, SHS exposure was positively associated with higher concentrations of urine NNAL in the entire population. In addition, women exposed to SHS were at a higher risk for T3 urine NNAL concentrations, independent of exposure place, while men were only at a higher risk only when exposed to SHS at the workplace. The null associations in males at home and in public places might be partly due to relatively higher levels of urine NNAL concentration in the male SHS non-exposure group.
The National Health Promotion Act of the Republic of Korea stipulated smoke-free policies for SHS exposure in public places [32]. In 1995, designated smoking areas in public places were established. In 2010, many outdoor areas, such as plazas, bus stations, and parks, were designated as outdoor smoke-free areas. In 2012, a smoking ban in the indoor areas of large restaurants was introduced, and finally, in 2015, indoor smoking was completely regulated for all restaurants [32].
According to a previous study that surveyed the attributable fraction of smoking to lung cancer in Korean women, the proportion of eversmoking-attributable lung cancer was 8.1%, while that attributable to SHS exposure among non-smoking women was 20.5% [33]. In addition, Asian women are more likely to be exposed to SHS, and the attributable burden of SHS exposure is also relatively higher [34]. This study also showed that in women, regardless of SHS exposure place, all ORs for the highest NNAL group (T3) were higher in men. In particular, exposure at home was 6 times higher with respect to the adjusted ORs for T3 than that of the non-exposure group in women. Thus, public health prevention efforts are needed regarding exposure to SHS in public places, including homes, especially among women. According to a recent Korean study [35], the prevalence of SHS exposure in Korea decreased from 2008 to 2018. However, widening health inequalities related to SHS exposure by education level have been reported [35]. Therefore, it is necessary to continuously implement tobacco control policies to reduce inequalities in SHS exposure.
This study had several limitations. First, causality is difficult to conclude because this study was cross-sectionally designed without follow-up. Second, because the data of this study were based on self-reported questionnaires, we could not exclude the possibility of reporting or recall biases. Therefore, the actual number of SHS exposures may differ. Third, because secondary data from the KNHANES were used, other factors that could affect NNAL concentrations were not considered.
Despite these limitations, this study has several strengths. Above all, our study is derived from a population-based sample with a complex survey design aimed at generating representative Korean population estimates. To the best of our knowledge, this is the first Korean study to investigate urine NNAL concentrations resulting from SHS exposure using national representative data. In addition, the actual NNAL concentration measurements are also a strength of this study.
In conclusion, we found that the mean urine NNAL concentration in the SHS exposure group was significantly higher than that in the non-exposure group after adjusting for several variables using national representative data. Exposure to SHS was associated with a higher risk of elevated urine NNAL concentrations in non-smokers. Therefore, the development of improved public health policies and preventive SHS exposure monitoring by medical professionals and the government is needed in light of our findings.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no.,2021R1C1C1003633).
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.21.0073. Supplement 1. Nonsmoker participant characteristics according to secondhand smoke exposure by sex.
Nonsmoker participant characteristics according to secondhand smoke exposure by sex