A Positive Association between the Atherogenic Index of Plasma and White Matter Hyperintensity
Article information
Abstract
Background
White matter hyperintensity (WMH) is a risk factor for dementia and ischemic stroke. The atherogenic index of plasma (AIP) is a simple and cost-effective marker for the prediction of various vascular diseases. In this study, we evaluated the relationship between AIP and WMH in adults without cerebrovascular accidents.
Methods
We analyzed the data of 281 adults, aged ≥26 years, who underwent brain magnetic resonance imaging (MRI) at the health promotion center of an education hospital between January 2014 and December 2018. Participants were divided into three categories according to tertiles of the AIP scores (T1: <0.20; T2: 0.20–0.48; and T3: >0.48). WMH was defined as a modified Fazekas scale score of 1–3 on brain MRI. A cubic spline curve was used to determine the linearity of the relationship between AIP and WMH. Multiple logistic regression analysis was used to evaluate the relationship between the AIP and WMH.
Results
The prevalence of WMH was 45.7% in T1, 57.0% in T2, and 66.0% in T3 (T3 vs. T1, P for post-hoc analysis=0.005). The increased odds of WMH were associated with increased AIP. The odds ratio (OR) with a 95% confidence interval (CI) for WMH of T2 and T3 compared with T1 were 1.57 (0.88–2.80) and 2.30 (1.28–4.14), respectively. After adjusting for confounding variables, the OR with a 95% CI for WMH in the T2 and T3 groups vs. the referent T1 were 1.55 (0.76–3.13) and 2.27 (1.06–4.84), respectively.
Conclusion
AIP is independently and positively associated with WMH in a healthy population.
INTRODUCTION
White matter hyperintensity (WMH) refers to white matter lesions with a signal intensity brighter than the surrounding white matter on the fluid attenuation inversion recovery (FLAIR) sequence of brain magnetic resonance imaging (MRI) [1]. WMH usually occurs with microvascular ischemic changes in the cerebrovascular vessels [2]. Although WMH is commonly found in older adults, the clinical manifestations of WMH vary from asymptomatic to cognitive impairment, dementia, or cerebrovascular accident (CVA), including ischemic stroke and transient ischemic attack [3]. Therefore, early detection and management of cerebrovascular diseases should be emphasized. Although brain MRI is the most precise and accurate non-invasive method for investigating cerebral microvascular diseases, including WMH, it is time-consuming and expensive. Hence, it is not routinely performed in clinical practice [4]. Much effort has been made to develop cost-effective and simple surrogate markers for the prediction of cerebral microvascular diseases [5].
Chronic inflammation and insulin resistance worsen the lipid profile, which contributes to atherosclerotic complications [6]. Recently, the atherogenic index of plasma (AIP), the base 10 logarithm of the ratio of triglycerides to high-density lipoprotein (HDL) cholesterol, has been developed [7]. It has been recognized as a useful predictive marker for plasma dyslipidemia and its related diseases, such as CVA, coronary heart disease, hypertension (HTN), diabetes mellitus (DM), metabolic syndrome, chronic kidney disease, and hyperuricemia [8,9]. Despite its correlation with atherosclerotic systemic diseases, there is limited evidence supporting the relationship between AIP and cerebral microvascular changes, especially in the Korean population. Therefore, we aimed to verify the association between AIP and WMH in a sample of Korean adults without CVA.
METHODS
1. Study Population
This study included data from 304 adults, aged >26 years, who underwent MRI imaging at the health promotion center of an education hospital between January 1, 2014, and December 31, 2018. We excluded 23 participants who had a history of CVA. In total, we analyzed data from 281 participants. All the participants provided written informed consent. The study was approved by the Institutional Review Board (IRB) of Nowon Eulji Medical Center (IRB approval no., 2021-03-026-001).
2. Atherogenic Index of Plasma Assessment
Blood samples were collected from the cubital vein after at least 10 hours of fasting. Fasting plasma glucose (FPG), serum total cholesterol, triglyceride, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, glycosylated hemoglobin (HbA1c), insulin, and high-sensitivity C-reactive protein (hsCRP) levels were measured using an ADVIA XPT Analyzer (Siemens, Erlangen, Germany). The AIP was calculated using the following equation [10]: AIP=log10 [triglycerides/HDL cholesterol].
We classified participants into three groups according to AIP tertiles as follows: T1, <0.20; T2: 0.20–0.48; and T3, >0.48.
3. White Matter Hyperintensity Assessment
Each MRI study included FLAIR imaging. Visual ratings of periventricular WMHs were performed by a experienced physician who was blinded to the clinical details. A simple, modified Fazekas rating scale was used for scoring. The modified Fazekas scale ranges from mild to severe, as follows [11]: (1) Fazekas scale 1: punctate lesions in the deep white matter with a maximum diameter of 9 mm for a single lesion, and 20 mm for grouped lesions. (2) Fazekas scale 2: early confluent lesions of 10–20 mm single lesions and >20 mm grouped lesions of any diameter, and no more than connecting bridges between the individual lesions. (3) Fazekas scale 3: single lesions or confluent areas of hyperintensity ≥20 mm in diameter.
WMH was defined as a Fazekas scale score of 1 to 3 on the FLAIR sequence of the brain MRI images.
4. Covariates
Height (m) and weight (kg) were measured to the nearest 0.001 m and 0.1 kg, respectively. Body mass index (BMI) was calculated by dividing weight by the square of height (kg/m2). We defined obesity as BMI ≥25 kg/m2 according to the definition of the Korean Society for the Study of Obesity [12]. After at least 30 minutes of rest, systolic blood pressure (SBP, mm Hg) and diastolic blood pressure (DBP, mm Hg) were measured in the sitting position.
Smoking status was categorized as current smoker or non-smoker. Participants with alcohol drinking status were categorized as current drinkers. White blood cell and platelet counts in whole blood were measured using an ADVIA 2120 analyzer (Siemens).
HTN was defined as SBP ≥140 mm Hg, DBP ≥90 mm Hg, or treatment with antihypertensive medications according to the criteria of the 7th Joint National Committee [13]. According to the 2020 American Diabetes Association criteria, we defined DM as an FPG ≥126 mg/dL, HbA1c ≥6.5, and treatment with anti-diabetic medications [14].
5. Statistical Analysis
All data were presented as means±standard deviations or medians (25th–75th percentile) for continuous variables, and as numbers (%) for categorical variables. Analysis of variance or Welch’s test was used to compare differences in the continuous variables among the three AIP tertiles, and the chi-square test was used to compare differences in categorical variables.
A cubic spline curve was used to determine the linearity of the relationship between AIP and WMH. The odds ratios (OR) and 95% confidence intervals (CI) for WMH of the T2 and T3 groups compared with the reference T1 group were calculated using multiple logistic regression analysis.
All statistical analyses were performed using the IBM SPSS software ver. 25.0 (IBM Corp., Armonk, NY, USA) and R ver. 4.0.3 (2008; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P<0.05.
RESULTS
1. Clinical Characteristics of the Study Population
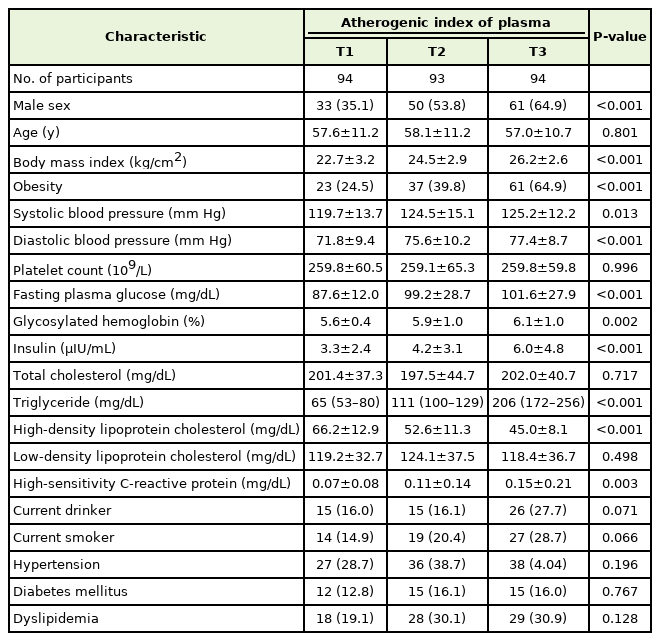
The clinical characteristics of the study population are summarized in Table 1. The proportion of men increased as the AIP tertile increased. There were no significant differences in average age between the groups. The mean values of BMI, SBP, DBP, FPG, HbA1c, serum insulin, hsCRP levels, and the median value of serum triglyceride levels increased as the tertiles of AIP increased. The mean HDL cholesterol level decreased as the AIP tertile increased. The proportion of obese participants was highest in T3, followed by T2 and T1.
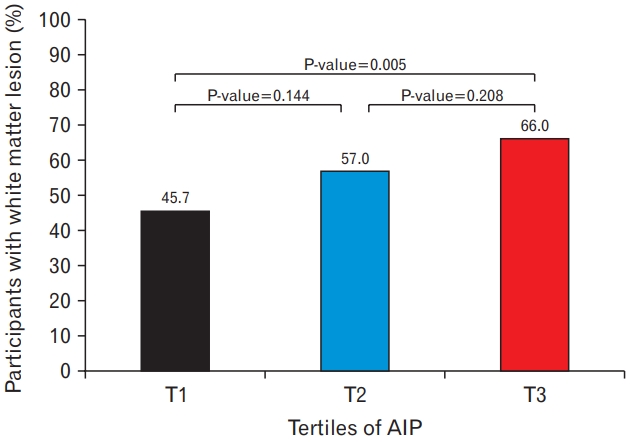
Figure 1 shows the percentage of participants with WMH according to the AIP tertiles. The prevalence of WMH was 45.7%, 57.0%, and 66.0% at T1, T2, and T3, respectively, with significant differences between the T1 and T3 groups (P-value for post-hoc analysis=0.005).
2. Relationship between Atherogenic Index of Plasma and White Matter Hyperintensity
A linear relationship between AIP and the odds of WMH was confirmed in the analysis using the cubic spline model (Figure 2). Increasing odds of WMH were shown as the AIP increased.
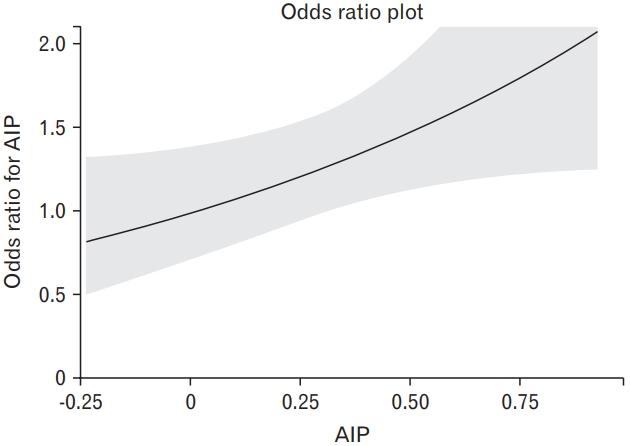
The cubic spline curve depicting the relationship between the atherogenic index of plasma (AIP) and the odds ratio for white matter hyperintensity.
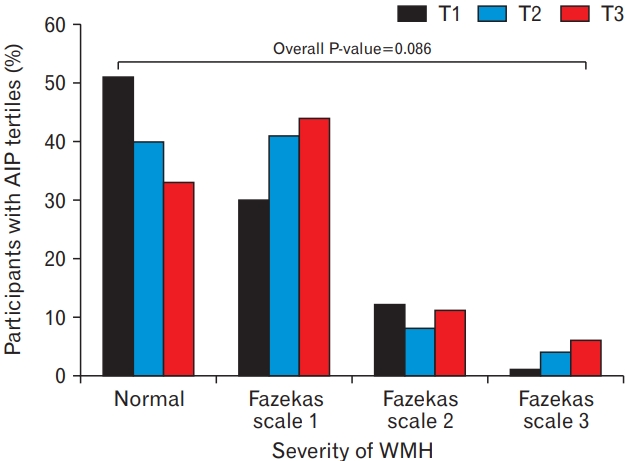
Table 2 presents the results of the multiple logistic regression analysis of the relationships between AIP and WMH. The OR (95% CI) for WMH of T2 and T3 compared with T1 was 1.57 (0.88–2.80) and 2.30 (1.28–4.14), respectively. After adjusting for confounding variables, including sex, age, obesity, current smoker, current drinker, HTN, DM, serum LDL cholesterol levels, whole blood platelet counts, and serum hsCRP levels, the adjusted OR with 95% CIs for WMH of T2 and T3 versus T1 were 1.55 (0.76–3.13) and 2.27 (1.06–4.84), respectively. Figure 3 shows the percentage of participants with AIP tertiles according to WMH severity. Although it was not statistically significant, normal white matter showed the highest proportion in the T1 group, whereas Fazekas scales 1 and 3 showed higher prevalence as tertiles increased.
DISCUSSION
We found that AIP was independently and positively associated with WMH in an apparently healthy population. This relationship persisted even after adjusting for confounding factors. Since small and dense LDL cholesterol is a major factor in the development of atherosclerosis, and is technically less convenient to measure, AIP is a potentially cost-effective alternative surrogate marker for the assessment of WMH.
AIP is independently associated with the occurrence of ischemic stroke [8,15]. Wang et al. [8] demonstrated a significant and positive association between AIP and ischemic stroke. The prevalence of ischemic stroke showed a substantially increasing trend with increasing AIP quartiles. Woo et al. [15] reported that elevated triglyceride levels and reduced the HDL level ratio (THR), and may be associated with intracranial atherosclerosis and a higher risk of early recurrent stroke events. However, their results did not demonstrate a significant association between THR and WMH. Most previous studies were dedicated to confirming the relationship between AIP and ischemic stroke [8,15]. Only a few studies investigated the relationship between AIP and WMH. Compared with previous studies, our study was based on the results of a relatively healthy population without CVA from screening examinations. Considering that WMH is a risk factor for incident ischemic stroke, our findings that AIP was related to WMH even in individuals without CVA support the results of previous studies [16,17].
While we could not explain the exact mechanism by which AIP is related to WMH, possible explanations include insulin resistance and chronic inflammation [16]. Higher serum triglyceride levels reduce insulin sensitivity and islet beta-cell function via superoxide dismutase [18]. Moreover, hypertriglyceridemia and low HDL cholesterolemia are usually accompanied by insulin resistance because insulin affects very-low-density lipoprotein (VLDL) and HDL cholesterol metabolism. Insulin resistance plays an important role in VLDL metabolism and increased hepatic triglyceride synthesis [19]. Increased VLDL triglyceride synthesis is linked to increased hepatic apolipoprotein (Apo) B-100 production [20]. This metabolism results in hypertriglyceridemia, which is reflected by increase in VLDL, Apo B-100, and decrease in HDL concentrations [21]. Park et al. [22] reported that triglycerides and LDL cholesterol correlated with the homeostasis model assessment of insulin resistance and beta-cell function, and thus, is a useful marker for predicting insulin resistance. Therefore, insulin resistance may affect the association between AIP and WMH.
Chronic inflammation is a major pathophysiological factor affecting metabolic syndrome and insulin resistance. Elevated triglyceride levels contribute to triglyceride-rich lipoproteins. Apo C-III, as a potent modular of plasma triglyceride levels, affects the pathway that activates necrosis factor-beta and increases inflammatory components such as vascular cell adhesion molecules (VCAM1), leading to the development of fatty streaks and advanced atherosclerosis [23]. Apo C-III is a glycoprotein synthesized by the liver and intestines. This glycoprotein affects the increase in triglyceride levels and stimulation of VLDL synthesis. VCAM1 is a membrane-bound adhesion receptor that plays a critical role in leukocyte accumulation [24]. The link between chronic inflammation and insulin resistance could be a possible explanation for the relationship between AIP and WHM.
The relationship between triglyceride levels and blood pressure should also be considered. Triglycerides are regarded as an independent risk factor for the early development of endothelial dysfunction [25]. As endothelial dysfunction and HTN have a bidirectional relationship, an increased serum triglyceride level may contribute to the elevation of blood pressure. Moreover, the relationship between triglycerides and blood pressure exists among participants with lower levels of circulating CD34-positive cells which have a beneficial role in endothelial repair [26].
HDL cholesterol exhibits anti-atherosclerotic effects by transporting cholesterol from peripheral vessels to the liver [27]. HDL cholesterol is also antioxidative. This is mediated by inhibiting the oxidation of LDL cholesterol, which results in the reduced cellular uptake of oxidized LDL cholesterol by the monocyte macrophage system [28]. In addition, HDL provides a pathway for detoxification or excretion of lipid peroxidation products, derived from oxidized LDL cholesterol, by accepting hydroperoxides from oxidized membranes [29]. In contrast, hypertriglyceridemia and low HDL cholesterolemia contribute to the development of WMH.
Our study had several limitations. First, we could not verify the causal relationship between AIP and WMH because of the retrospective cross-sectional design of this study. Further cohort studies are needed to elucidate the causal relationship between AIP and the incidence of WMH. Second, specific information regarding the medications used by patients with HTN, DM, or dyslipidemia was not evaluated. However, we adjusted for the major risk factors of atherosclerosis to minimize information bias. Third, the results could not be generalized because we analyzed only Korean data. As the Korean population has a higher prevalence of ischemic stroke than Westerner populations [30], the positive relationship between AIP and WMH may be weaker in other ethnicities. Finally, the relatively small sample size of this study may limit its generalizability. Future large-scale, multi-ethnic studies to confirm the association between AIP and WMH in the general population are warranted.
In conclusion, our analyses showed that AIP was independently and positively associated with WMH in an apparently healthy population. AIP could be a cost-effective method for screening and identifying individuals with WMH who require early intervention.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We thank Hye-Jung Choi from the Department of Family Medicine for his help in data collection and processing.