 |
 |
- Search
| Korean J Fam Med > Volume 43(4); 2022 > Article |
|
Abstract
Background
The use of topical antibiotics (TA) for prophylactic purposes after clean dermatologic procedures (CDP) is generally not recommended, and the prescription of TA needs to be individualized in consideration of each patient’s situation and underlying disease. The aim of this study was to determine the proportion of patients who underwent CDP in outpatient settings and were prescribed TA inappropriately, as well as the factors that may affect the prescription of TA.
Methods
Outpatient visits coded for CDP were selected using claims data from the Health Insurance Review and Assessment Service in 2018. Of these, patients receiving TA prescriptions were classified as having inappropriate TA use, and the proportion was estimated through technical analysis. A logistic regression analysis was used to identify factors influencing inappropriate prescriptions.
Results
Data were analyzed using 423,651 visits, and TA was prescribed for approximately 1.9% of the visits. TA usage was higher among women (2.0%), 0–19 years of age (2.2%), medical aid (2.2%), clinic settings (2.4%), and metropolitan areas (2.0%). TA was prescribed more frequently in urology (8.6%), pediatrics (5.0%), and dermatology (4.2%) than in other specialties.
Conclusion
The prescription rate of TA after CDP was 1.9% using the 1.4 million patient sample from the national health insurance claims data in Korea, which is equally weighted to represent 50 million people. Although the proportion of inappropriate TA prescriptions in Korea is lower than that in other nations, it cannot be overlooked because of the large number of cases. Efforts to improve quality are required to reduce the number of inappropriate prescriptions.
There are a number of dermatological procedures and surgeries for the diagnosis and treatment of skin diseases. Wounds inevitably occur after a procedure or surgery, and wound management to prevent secondary infection and ensure proper healing is very important [1]. Infection is the most common impediment to normal wound recovery, and a number of pre-and post-management procedures are performed to prevent infection and help rapid recovery. Disinfectants, such as chlorhexidine and povidone, and antibiotic ointments, such as mupirocin and bacitracin, are most used in pre-and post-management, respectively [2,3].
However, excessive use of topical antibiotics (TA) leads to antibiotic resistance, allergic contact dermatitis, anaphylaxis, and an increase in unnecessary medical costs [1]. In a practical view, the expected outcome of using prophylactic TA is also insignificant [4].
Most dermatological procedures and surgeries are relatively simple, the treatment site is usually clean or clean-contaminated, and the incidence of secondary infection is very low [4,5]. There is insufficient evidence for using a TA to treat a clean lesion caused by a procedure or surgery, and the American Academy of Dermatology and the American Academy of Family Medicine recommend the routine use of TA on a surgical wound [6,7].
When comparing TA and petrolatum/paraffin products, studies have shown that petrolatum/paraffin products could be a more appropriate treatment if the wound in the treatment area is not severe. Several studies have shown that there is no significant difference between the two products in the prevention of infection at the wound site [4], and petrolatum-based products are safer and have relatively superior clinical results [1].
However, TA are recommended for dirty or infected wounds, immunocompromised patients, and patients with a high risk of infection. In other words, the prescription of TA needs to be individualized in consideration of each patient’s situation and underlying disease [3,5].
Many studies have reported high rates of antibiotic use regardless of the indications. Few studies have measured the prescription rates of TA for prophylaxis following dermatological procedures. Levender et al. [8] reported that an estimated 212 million dermatologic procedure (CDP) were performed between 1993 and 2007, and TAs were used in 10.6 million (5.0%) procedures. Dermatologists performed 63.3% of dermatologic surgical operations, with 8.0 million (6.0%) of these procedures including TA prophylaxis. However, there was a significant decrease in the use of TA prophylaxis from 1993 to 2007, indicating that providers’ practices are changing to meet the standard of care [8]. In a study by Lapolla et al. [9], the National Ambulatory Medical Care Survey found a substantial decrease in TA linked with dermatologic surgery from 1993 to 2007, but it can be seen that it remains around 4%. Fathy et al. [10] showed that patients visited by dermatologists received an estimated 10.2% and 5.75% TA prescriptions per year for biopsies and excisions, respectively; the prescription rate was found higher among dermatologists compared to non-dermatologists. Barbieri et al. [11] found that prescriptions for oral antibiotics in conjunction with benign excisions increased from 2.9% to 4.4% of visits, malignant excisions from 4.2% to 6.3%, and Mohs surgery from 9.9% to 13.8% of visits.
In studies conducted in the United Kingdom, Ferguson et al. [12] reported that antibiotics prescribed regardless of all indications were 44.5 million in 1995 and 34.2 million in 2000; Eady et al. [13] found that the antibiotics prescribed for the treatment of acne were 3.3 million in 1995 and 2.6 million in 2000, so a high rate of antibiotic prescriptions could be considered a phenomenon that is observed in many countries.
Although studies have been conducted in the United States, the United Kingdom, and Germany, there has been no research in Asian countries, including South Korea. Therefore, this study aimed to understand the status of prophylactic TA use after a CDP in South Korea. In addition, this study attempted to assess the factors that may influence the use.
This study used the 2018 National Patient Sample (NPS) claims data from the Health Insurance Review and Assessment Service (HIRA) (serial number: HIRA-NPS-2018-002). The HIRA-NPS is a populationrepresentative sample dataset that provides demographic information, medical use, prescriptions, and injury reports for 1.4 million patients out of a total population of 50 million.
The purpose of the research was to evaluate the use of TA among patients who received CDP in outpatient settings. The TA and CDP considered in this study were selected from TA and dermatological procedures that were mentioned in previous studies. [1,4,5,8]. The operational definition of CDP is based on the fact that dermatologic surgery wounds do not meet the criteria for the CDC classification scheme of surgical wounds, and several dermatology studies used the expanded definition of either clean or clean-contaminated wounds to describe dermatology surgery wounds [14,15]. The operational definition of TA is TA prescribed for prophylactic purposes following dermatologic surgery. HIRA-NPS data were then queried to select outpatient visits that were coded for a CDP (Appendix 1). The selection process for this study is illustrated in Figure 1. The number of outpatient visits coded for the CDPs was 691,578. Procedures were confirmed by searching the International Classification of Diseases, 10th Revision for all procedure codes related to the skin and subcutaneous tissues [8]. Of these, 239,772 visits that included infection codes (Appendix 2) were excluded. A total of 25,166 visits coded for more than two procedures were excluded. We excluded 2,990 visits with invalid data (e.g., missing values). We analyzed 14,121,529 visits by assigning a weight of 33.3 to the extracted 423,651 visits.
This study was exempted from ethical approval by the Chungnam National University Institutional Review Board (202005-SB-042-01).
The chi-square test was performed to assess differences in the degree of prescription for TAs depending on the general characteristics: sex (male, female), age (0–19, 20–39, 40–64, 65, and older), insurance type (national health insurance [NHI], medical aid), location of a medical institution (metropolitan area, non-metropolitan area), medical institution type (tertiary hospital, general hospital, hospital, clinic), specialty (dermatology, orthopedic surgery, general surgery, general practitioner, plastic surgery, emergency medicine, neurosurgery, internal medicine, obstetrics and gynecology, urology, family medicine, pediatrics, etc.). Our classification of TAs included mupirocin, neomycin, gentamicin, betamethasone/gentamicin, and betamethasone/clotrimazole/gentamicin. Lastly, to identify factors affecting the prescription of TA during outpatient visits that are coded for the CDP, we conducted logistic regression with the use of TAs as the dependent variable, and sex, age, insurance type, location of the medical institution, medical institution type, and specialty as independent variables. The variance inflation factor was 1.1, and the condition index was <10 as a result of evaluating multicollinearity among the independent variables.
Multiple logistic regression analyses were conducted to identify the factors associated with TA use during outpatient visits for CDP. Model 1 was adjusted for sex, age, insurance type, location of medical institutions, and medical institution type, whereas model 2 was adjusted for sex, age, insurance type, location of medical institutions, medical institution type, and specialty. The results of model 1 were parallel to those of the univariate analysis in Table 1.
The total number of visits prescribed for TAs following the CDP was 1.9%. TAs were prescribed to 1.9% of men and 2.0% of women who underwent CDP. The TA prescription rates for each age group were 2.2, 2.1, 1.8, and 1.8, respectively. A total of 1.9% of the patients with NHI were prescribed TAs, whereas 2.2% of those with medical aid were prescribed TAs. A total of 1.7% and 2.0% of visits in non-metropolitan areas and metropolitan areas, respectively, were prescribed TAs. TA prescriptions were linked to 1.9%, 1.5%, 0.8%, and 2.4% of tertiary hospitals, general hospitals, hospitals, and clinics, respectively. Therefore, patients with medical aid, patients who visited a clinic, and patients in metropolitan areas were more likely to receive TAs after CDP. In order of descending frequency by specialty, TA prescriptions were as follows: urology (8.6%), pediatrics (5.0%), and dermatology (4.2%) (Table 1).
Model 1 showed results similar to those of univariate analysis, while model 2 presented slightly different results, particularly in sex, age, and medical institution type. In model 2, a lower odds ratio was observed in women (odds ratio [OR], 0.96; 95% confidence interval [CI], 0.95– 0.97) than in men. Patients in the 20–39 age group (OR, 1.15; 95% CI, 1.13–1.16) had a greater risk than other age groups. The risk increased in patients who had visited a general hospital (OR, 1.17; 95% CI, 1.15– 1.20), rather than in those who had visited other types of medical institutions (Table 2).
The prophylactic use of TAs for clean or clean-contaminated wounds following dermatological procedures is no longer recommended by guidelines. In this study, the status of TA used for these purposes was investigated, as well as the factors influencing the use of TAs. Data from 423,651 visits were analyzed, and TAs were prescribed in approximately 1.9% of the visits. Patients with medical aid, people who use medical facilities in metropolitan areas, especially general hospitals, and urologists and dermatologists, have a higher possibility of receiving inappropriate TA prescriptions.
This research builds upon prior studies that reported antibiotic use between 2.9% and 13.8% of the CDP.8-11) In this study, the prescription rate of TAs after the CDP was 1.9%.
The most inappropriate TAs used by medical institution types were clinics (2.5%), tertiary hospitals (1.9%), general hospitals (1.5%), and hospitals (0.8%). The rate of inappropriate TA prescriptions in clinics is unusually high compared with other types of medical institutions. This may be due to the different nature of primary care in Korea compared to other countries with strong primary care, where there is little difference in quality between specialists and primary care. In Western countries, clinics usually care for outpatients, while hospitals primarily care for inpatients. A patient must first consult a primary physician and acquire a referral letter before visiting a doctor at the hospital. Following the conclusion of the medical care, the patient was released back to his or her primary physician. Thus, clinics and hospitals have a collaborative relationship [16]. In contrast, the Korean government allows clinics to provide inpatient facilities and hospitals to offer a wide scale of services to outpatients [17,18]. Patients in Korea do not require a referral slip to access either clinics or hospitals [19]. Therefore, the competitive nature of the clinical–hospital relationship seems to have resulted in a strong motivation to draw and retain as many patients as possible to maximize their income by offering the most available services. Whether it was effective or appropriate may not necessarily be important.
The rate of inappropriate TA use in hospitals is noticeably lower than that in other medical institutions. If most patients in the hospital are elderly or poor, they may have been asked to purchase an over-thecounter antibiotic ointment from a relatively inexpensive pharmacy after the CDP. If so, the prescription rate for TAs would be low because the data only collected information on prescribed drugs. In general, public hospitals and health facilities provide uninsured coverage at lower retail prices than private institutions, which explains why public hospitals handle a higher proportion of disadvantaged and medical aid patients [17]. Separating tasks of prescribing and dispensing between doctors and pharmacists, which was adopted in 2000, was seen by doctors as jeopardizing their economic interests and resulted in strikes. Separation reform had both positive and negative consequences. In terms of antibiotic prescriptions, the number of antibiotics prescribed by doctors decreased after the reform. However, without incentives (income) under the new system, physicians tend to prefer expensive branded drugs to inexpensive ones [20,21]. Therefore, physicians at hospitals may prefer not to prescribe expensive antibiotics to the elderly or medical aid patients.
The number of medical institutions varies by geography, which explains the improper use of TAs in metropolitan regions. Medical personnel are heavily concentrated in Seoul and the six largest cities because of the widening income gap between major cities and rural areas, as well as the significant concentration of healthcare facilities and communities in these large cities. Despite accounting for only about 47% of the country’s total population, Seoul and the six major cities are home to 51.4% of all general practitioners and 73.3% of specialists (2005 data) [17].
Inappropriate TA use is high among medical aid insurers because the rate of use of medical services varies by the type of medical coverage. In 2018, it was estimated that 62.4% of national health insurers and 72.0% of medical aid insurers used medical services [22]. Inappropriate use of TAs is high among medical aid patients due to their relatively high use of medical services. Noh et al. [23], who analyzed factors related to outpatient utilization in Korea, reported that medical aid recipients showed higher outpatient utilization than NHI beneficiaries. The higher outpatient utilization among medical aid patients, according to Shin et al. [24], can be attributed to two factors. The first is the overuse of health care services, which is exacerbated by the fact that medical aid is either free or significantly less expensive than health insurance. Second, because medical aid is reimbursed on a fee-for-service basis, healthcare practitioners may have prescribed excessive treatment [24]. Health insurers are subjected to 20% and 30% co-payments for inpatient care and outpatient care, respectively, while the Medical Aid Program covers both the insurance premium and co-payments for medical aid recipients [25,26]. According to Gwatkin [27], lower-income groups in high-income nations use health care more frequently as a benefit of their social security system.
The relatively high rate of inappropriate use of TAs in pediatric patients may be due to false medical beliefs. Two of the six reasons that may cause overutilization/overtreatment, summarized by Kazemian et al. [28], reflect that incorrect medical beliefs lead to inappropriate medical care. The first reason is that the beliefs of patients and caregivers are that more testing and use of drugs provide better care. The second reason is the attitude of the doctor, who cannot reject the wrong requests from patients [28-30]. A study by Seock and Gun [31] also states that there is a prevalent belief that faster and more medical tests and treatments are better in Korea. For example, some patients require injections for a simple cold and go to the hospital for so-called nutritional injections even if they experience fatigue [31].
Interestingly, urology prescribed the most TAs compared with other specialties (Supplement 1). The answer may lie in the history of urology in South Korea. Before the department of urology was recognized as an independent subject in 1954, urological diseases, including sexually transmitted diseases, were treated by the Department of dermatologyurology, internal medicine, or surgeon [32,33]. Furthermore, the national regulations on sexually transmitted diseases established in 2000 authorized urology, along with dermatology, obstetrics, and gynecology departments, to treat and prevent sexually transmitted diseases [34]. As a result, there is no clear distinction between the department of urology and the department of dermatology, so even today, practitioners in the department of urology continue to treat dermatological diseases as well.
Although a relatively lower rate of TA use was observed, further investigation, such as a review of actual medical records, is needed to address the limitations of this study. This study has limitations, mainly in terms of methodology. The first limitation is that health insurance claim data only includes information about medical utilization (e.g., medication or use of medical supplies and equipment) covered by NHI. As data on patients who received uninsured medications or uncovered procedures could not be included, the total amount of TA use in the CDP may have been underestimated. The second limitation was that only the affecting factors available in the claim data could be considered, thus failing to consider other factors that could influence inadequate prescription. Third, the clinical adequacy of TA could not be considered. Although there is no information on the accuracy of the diagnosis of infectious diseases in the Korean health insurance claims data, there may be some inaccurate diagnosis codes.
In conclusion, using the 1.4 million patient sample from the NHI claims data, the prescription rate of TA after CDP was found to be 1.9%. While the proportion of inappropriate TA prescriptions is lower than in other countries, the high number of cases should not be underestimated. Quality assurance measures are required to minimize inappropriate prescriptions for TA.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.21.0139. Supplement 1. Cross-table between medical specialties and other independent variables.
Figure. 1.
Selection process of patients with clean dermatologic procedures. HIRA-NPS, Health Insurance Review and Assessment Service-National Patient Sample.
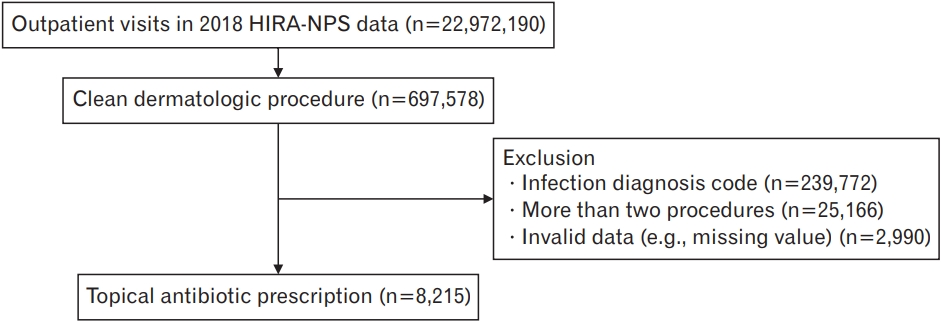
Table 1.
Comparison of the degree of prescription for topical antibiotics depending on the general characteristics
Table 2.
Logistic regression to identify factors affecting the prescription of topical antibiotics during outpatient visits that are coded for the clean dermatological procedure
REFERENCES
1. Nguyen JK, Huang A, Siegel DM, Jagdeo J. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg 2020;46:186-91.


2. Fathy R, Chu B, Singh P, James WD, Barbieri JS. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med 2021;36:238-9.




3. Totoraitis K, Cohen JL, Friedman A. Topical approaches to improve surgical outcomes and wound healing: a review of efficacy and safety. J Drugs Dermatol 2017;16:209-12.

4. Saco M, Howe N, Nathoo R, Cherpelis B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and meta-analysis. J Dermatolog Treat 2015;26:151-8.


5. Smack DP, Harrington AC, Dunn C, Howard RS, Szkutnik AJ, Krivda SJ, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment: a randomized controlled trial. JAMA 1996;276:972-7.


6. Choosing wisely [Internet]. Des Plaines (IL): American Academy of Dermatology Association; [date unknown] [cited 2021 Aug 2]. Available from: https://www.aad.org/member/clinical-quality/clinicalcare/wisely
7. Choosing wisely: don’t routinely use topical antibiotics on a surgical wound [Internet]. Leawood (KS): American Academy of Family Physicians; [date unknown] [cited 2021 Aug 2]. Available from: https://www.aafp.org/pubs/afp/collections/choosing-wisely/150.html
8. Levender MM, Davis SA, Kwatra SG, Williford PM, Feldman SR. Use of topical antibiotics as prophylaxis in clean dermatologic procedures. J Am Acad Dermatol 2012;66:445-51.


9. Lapolla WJ, Levender MM, Davis SA, Yentzer BA, Williford PM, Feldman SR. Topical antibiotic trends from 1993 to 2007: use of topical antibiotics for non-evidence-based indications. Dermatol Surg 2011;37:1427-33.


10. Fathy R, Chu B, James WD, Barbieri JS. The frequency of topical antibiotic use after biopsy and excision procedures among dermatologists and nondermatologists: 2006 through 2015. J Am Acad Dermatol 2020;82:1258-61.



11. Barbieri JS, Etzkorn JR, Margolis DJ. Use of antibiotics for dermatologic procedures from 2008 to 2016. JAMA Dermatol 2019;155:465-70.



12. Ferguson J. Recent trends in the prescribing of antibiotics. Prescriber 2001;12:59-62.
13. Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant?: implications of resistance for acne patients and prescribers. Am J Clin Dermatol 2003;4:813-31.

14. Garner JS. CDC guideline for prevention of surgical wound infections, 1985: supersedes guideline for prevention of surgical wound infections published in 1982 (originally published in November 1985): revised. Infect Control 1986;7:193-200.


15. Rogers HD. Reply. J Am Acad Dermatol [Internet] 2011 [cited 2021 Aug 2];65:203. Available from: https://www.jaad.org/article/S0190-9622(11)00008-9/pdf
16. Ock M, Kim JE, Jo MW, Lee HJ, Kim HJ, Lee JY. Perceptions of primary care in Korea: a comparison of patient and physician focus group discussions. BMC Fam Pract 2014;15:178.




17. Chun CB, Kim SY, Lee JY, Lee SY. Republic of Korea: health system review. Health Syst Transit [Internet] 2009 [cited 2021 Feb 25];11:1-184. Available from: https://apps.who.int/iris/bitstream/handle/10665/330337/HiT-11-7-2009-eng.pdf?sequence=5&isAllowed=y
18. Kwon S. Payment system reform for health care providers in Korea. Health Policy Plan 2003;18:84-92.


19. Song YJ. The South Korean health care system. Japan Med Assoc J 2009;52:206-9.
20. Kim HJ, Ruger JP. Pharmaceutical reform in South Korea and the lessons it provides. Health Aff (Millwood) 2008;27:w260-9.


21. Cho J. A study for the establishment of separation of prescription and dispensing [Internet]. Sejong: Korea Institute for Health and Social Affairs; 2001 [cited 2021 Mar 18]. Available from: https://www.kihasa.re.kr/en/publish/paper/research/view?seq=29848
22. National Health Insurance Corporation; Health Insurance Review and Assessment Service. Health insurance statistics [Internet]. Daejeon: Statistics Korea; [date unknown] [cited 2021 Mar 18]. Available from: https://www.kosis.kr
23. Noh JW, Kim KB, Park H, Kwon YD. Gender differences in outpatient utilization: a pooled analysis of data from the Korea Health Panel. J Womens Health (Larchmt) 2017;26:178-85.


24. Shin SM, Kim MJ, Kim ES, Lee HW, Park CG, Kim HK. Medical aid service overuse assessed by case managers in Korea. J Adv Nurs 2010;66:2257-65.


25. Kwon S, Lee TJ, Kim CY, World Health Organization; Regional Office for the Western Pacific; Asia Pacific Observatory on Health Systems and Policies. Republic of Korea health system review. Manila: World Health Organization, Regional Office for the Western Pacific; 2015.
26. National Health Insurance Service. NHI Program [Internet]. Wonju, National Health Insurance Service. [date unknown] [cited 2021 Feb 24]. Available from: https://www.nhis.or.kr/static/html/wbd/g/a/wbdga0403.html
27. Gwatkin DA. Initial country-level information about socio-economic differences in health, nutrition and population [Internet]. Washington (DC): World Bank; 2003 [cited 2021 Feb 24]. Available from: https://books.google.co.kr/books?id=qlbvzQEACAAJ
28. Kazemian A, Berg I, Finkel C, Yazdani S, Zeilhofer HF, Juergens P, et al. How much dentists are ethically concerned about overtreatment: a vignette-based survey in Switzerland. BMC Med Ethics 2015;16:43.




29. Katz MH, Grady D, Redberg RF. Undertreatment improves, but overtreatment does not. JAMA Intern Med 2013;173:93.


30. Torjuul K, Nordam A, Sorlie V. Action ethical dilemmas in surgery: an interview study of practicing surgeons. BMC Med Ethics 2005;6:E7.




31. Seock C, Gun P. What factors promote overtreatment in Korea?: causative considerations and solutions to overtreatment. Korean J Med Ethics 2016;19:375-89.

32. Park JY, Park K, Jeong SJ. History of Investigative and Clinical Urology and an analysis of published articles. Investig Clin Urol 2020;61(Suppl 1):S64-9.




33. Kee CD. The history of urology in Korea (1910-1945). Korean J Med Hist 1997;6:231-69.
Appendices
Appendix 1.
Clean dermatologic procedures
Appendix 2.
Infection diagnosis codes
- TOOLS







