Discussion on the Clinical Course of Adverse Effects after COVID-19 Vaccination: A Retrospective Analysis of Case Series in an Outpatient Department
Article information
Abstract
Background
A total of 8,303 individuals (4.3%) with adverse reactions (n=191,860) after vaccination developed serious conditions or died. Such health developments could cause people not vaccinated yet or waiting for a booster shot to become fearful of the vaccination.
Methods
The 3-month (July–September 2021) clinical data of 41 patients from the family medicine department of a single medical center were analyzed retrospectively to determine risk factors and to investigate the clinical course to identify the cause of symptoms in detail.
Results
A significant number of older adults aged over 50 years reported experiencing general weakness (P=0.026) but fewer incidences of fever than patients aged 50 years or younger (P=0.011). Eighteen of the 41 patients were requested to visit more than twice or consult a specialist. In 14 patients, the symptoms were explained by other medical causes.
Conclusion
The primary physician has a pivotal role in thoroughly evaluating patients who complain of adverseeffects after vaccination, considering the broad multitude of symptoms and medical conditions presented. To thoroughlyevaluate and appropriately advise patients with adverse reactions to their chosen vaccine, taking detailedmedical history and nutritional counseling are required to identify possible underlying causes, resolve symptoms,and educate them on self-care and regarding vaccines.
INTRODUCTION
The World Health Organization has defined an adverse event following immunization as “any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine.”
Vaccination is one of the most effective methods recommended in many countries to overcome the coronavirus disease 2019 (COVID-19) pandemic. As a result, several vaccines have been launched for inoculation against this viral infectious disease. Four drugs (AstraZeneca, Pfizer, Moderna, and Janssen) are currently being administered in South Korea.
The total number of adverse effects reported after vaccination was 191,860 in 46,474,326 people who received the vaccination within a 27-week period (February 26, 2021–September 4, 2021) [1]. Although 8,308 cases (4.3%) involved death or severe symptoms (death, anaphylactic shock, stroke, acute heart disease, coronary heart disease, pulmonary thromboembolism, deep vein thrombosis, septic shock, and any cases hospitalized in the intensive care unit), 183,552 cases (95.7%) were classified as not serious based on milder symptoms at the time of visit. Severe adverse events to total administration were reported as 0.01%–0.03% in the four brands of vaccines and at any age (total 0.02%, AstraZeneca 0.02%, Pfizer 0.01%, Moderna 0.01%, Janssen 0.03%). Only the ratio of severe adverse events to total adverse events was relatively higher in individuals aged >60 years (12–17 years: 1.65%, 18–29 years: 2.38%, 30–39 years: 3.37%, 40–49 years: 3.40%, 50– 59 years: 3.32%, 60–69 years: 4.44%, 70–79 years: 6.60%, and ≥80 years: 11.21%).
However, many people still fear receiving a booster shot because of uncertain clinical information or long-term prognosis. Therefore, this study was performed to gather information to help clinicians counsel patients who presented with only mild symptoms in the outpatient department.
Thus, this study aimed to provide support to primary physicians, in examining patients who complain of adverse effects after the administration of the COVID-19 vaccine and to discuss the possible causes and results of such phenomena.
METHODS
Data were collected from a single medical center for 3 months (July– September 2021). All patients met a family medicine doctor via the customer center at the family medical outpatient office to discuss their symptoms and obtain medical advice. Sex, age, ongoing health conditions, and medical history were evaluated. Medical tests, such as laboratory assessment, electrocardiography, and imaging studies, were requested by the doctor in charge after the first medical examination. Arrangements for follow-up or consultation with other specialists were made based on the test results. The cases were then reviewed to determine the realistic causes of the adverse event symptoms. All this information was collected and inspected retrospectively for analysis and to determine the relationships among the brand of vaccine, patient characteristics, symptoms, and laboratory data.
The IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The chi-square test was used to determine the relationship between each adverse effect and the brand of vaccine and the laboratory findings. Logistic regression analysis was used to analyze the relationship between age and symptoms. The statistical significance level was set at P<0.05.
RESULTS
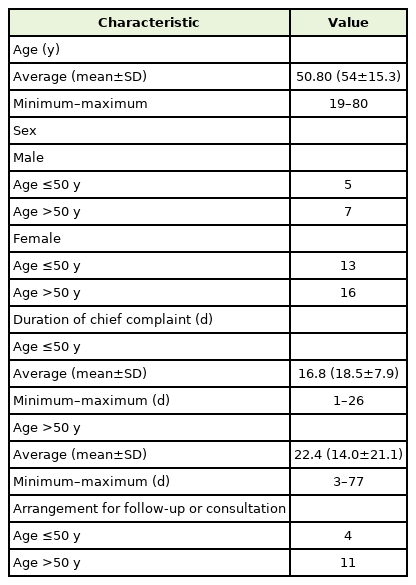
Forty-one patients who visited the hospital for consultation of their symptoms after vaccination were evaluated. Medical laboratory data were obtained for 38 patients. The average±standard deviation (SD) age of 12 male and 29 female patients was 50.8±15.3 years (minimum, 19 years; maximum, 80 years). Twenty-three patients were >50 years of age. In three patients, the initial symptoms had already evidently improved at the visit. Janssen was administered to two patients, Astra-Zeneca in 13 patients, Pfizer in 20 patients, and Moderna (Mo) in six patients (Figure 1). Thirty-seven patients presented for consultation on adverse effects after the first dose of the vaccine. The mean±SD duration from vaccination to visit was 19.9±16.7 days (minimum, 1 days; maximum, 77 days) (Table 1).
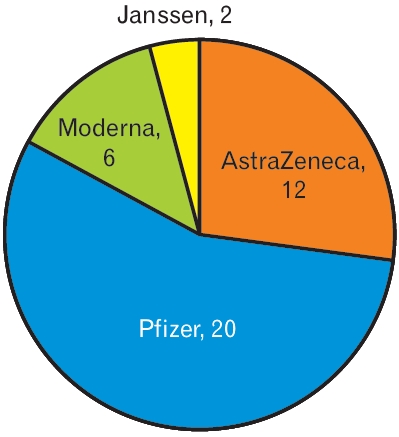
Distribution of patients who had adverse effects after administration of each brand of vaccine. 12 patients were administrated AstraZeneca. 20 patients were administrated Pfizer. 6 patients were administrated Moderna. 2 patients were administrated Janssen.
The most common symptom reported by patients after vaccination was a headache. Severe generalized weakness and skin lesions such as petechiae, bruising, or subcutaneous hemorrhage were also observed (Figure 2). Each vaccine brand had a different distribution of adverse effects (Figure 3). Skin lesions were the most common adverse effects in the AstraZeneca group. Headaches and chest pain were common in Pfizer vaccines, whereas cellulitis often occurs with Moderna vaccines. (Janssen were counted only as part of the total statistical calculation because of the small number of cases in which they were used.)
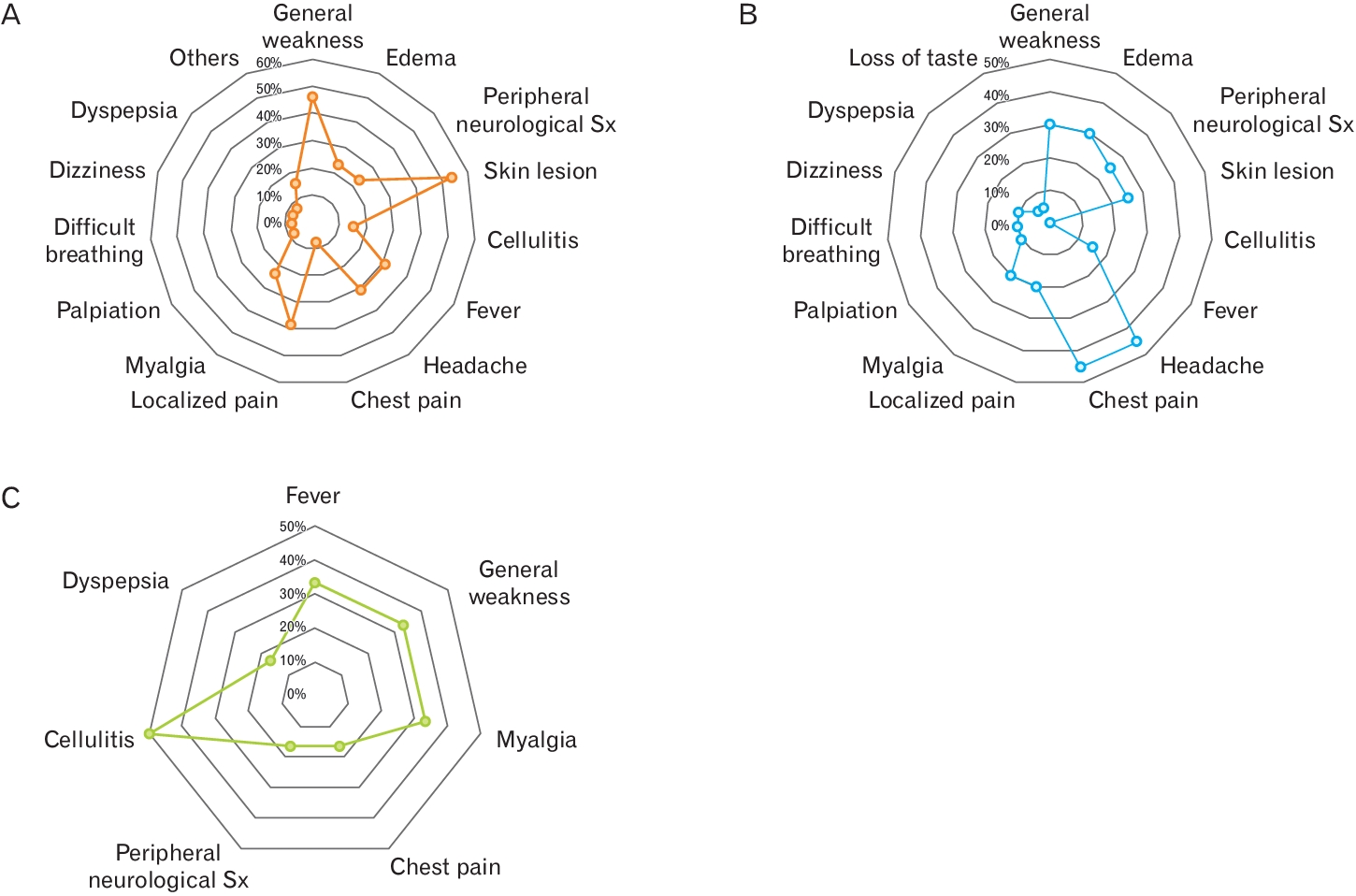
Frequency distribution of complaints of whom patients visited for examination about adverse events after vaccine administration. (A) AstraZeneca. (B) Pfizer. (C) Moderna. Sx, symptoms.
The chi-square test did not show a significant relationship between symptoms and each brand of vaccine or the number of doses, although each vaccine brand varied in the frequency of adverse effects. Sex and duration were also not significantly associated with symptoms.
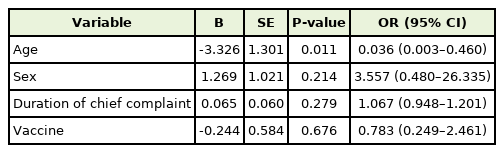
Age may be associated with general weakness and fever (Tables 2, 3). Logistic regression analysis showed that patients over 50 years of age experienced general weakness more frequently than younger patients. Older age was associated with a tendency to require follow-up or further consultation, but this was not statistically significant. In contrast, younger patients experienced fever more frequently than older patients. However, fever was not a major concern in most patients. Fever occurring shortly after vaccination is a commonly expected symptom in the public, and in most cases, fever resolves in 4 days.
Eighteen patients needed to make appointments more than twice or consult with other specialists (Tables 4, 5). The primary care doctor decided whether to return to the local clinic or follow up for each case. The conditions prioritized for follow-up were abnormal findings, such as those on electrocardiography, laboratory tests, and physical examination, or when special tests, such as endoscopy, needed to be conducted.

Basic states of patients who made consultations more than twice or were referred to another specialist
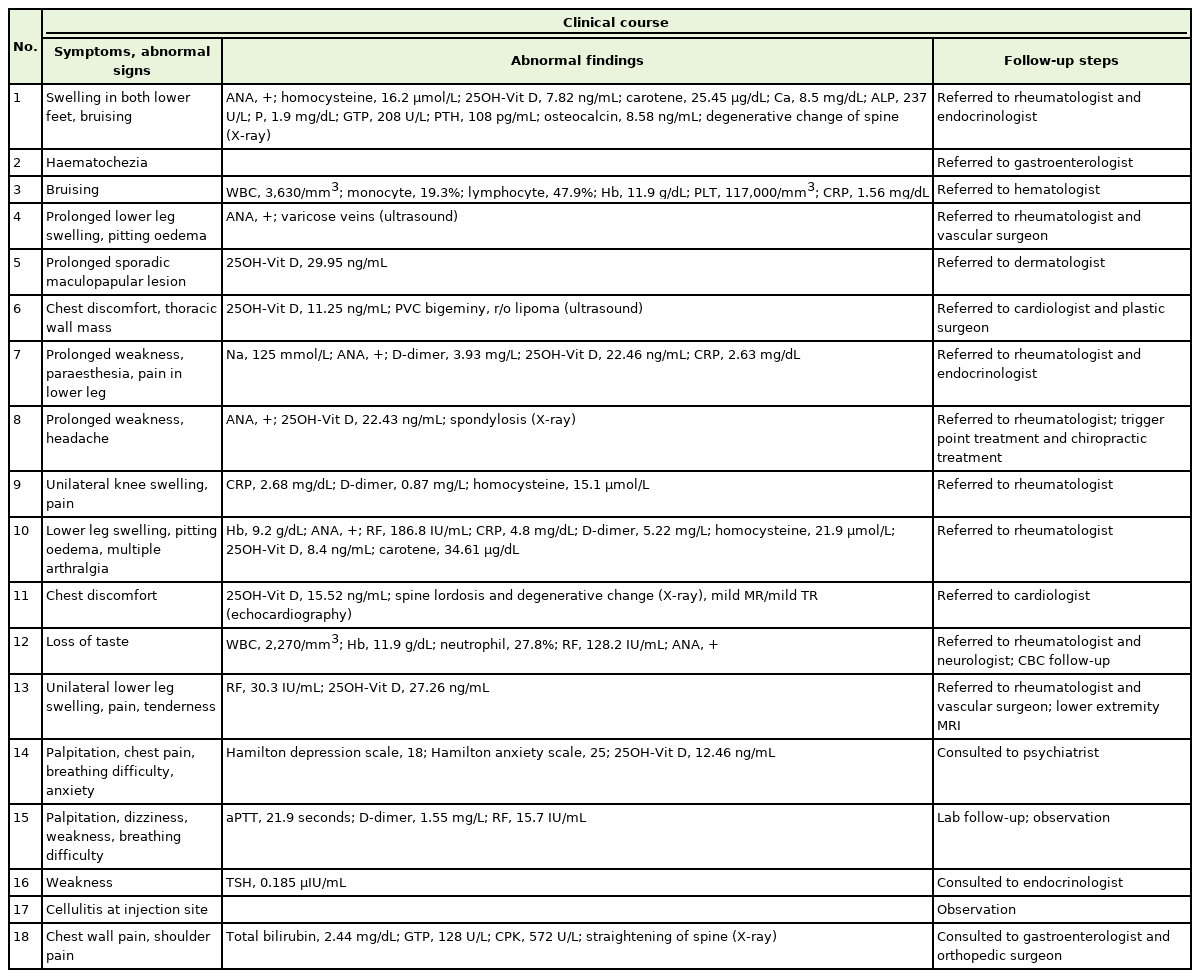
Clinical course of patients who made consultations more than twice or were referred to another specialist
Patient no. 1: Suspected sciatica upon physical examination and imaging. His leg pain improved dramatically after manual therapy and functional internal muscular stimulation. He is awaiting an examination by a rheumatologist and endocrinologist for positive antinuclear antibody and hyperparathyroidism.
Patient no. 2: He had no abnormal laboratory findings or colonoscopic lesions except for internal hemorrhoids.
Patient no. 3: His symptoms improved, and laboratory parameters normalized approximately 1 month later.
Patient no. 4: Varicose veins in both lower legs were identified as the source of pain after ultrasonography by a vascular surgeon. She was treated conservatively by a vascular surgeon and regularly checked by a rheumatologist.
Patient no. 5: She was prescribed a topical steroid agent by a dermatologist and was awaiting her next visit.
Patient no. 6: He was previously diagnosed with a symptomatic ventricular premature complex. However, he skipped taking propafenone medication for several days before the vaccination. The mass found in his chest wall appeared to be a lipoma on ultrasonography. His chest symptoms decreased after the reintake of antiarrhythmic agents. He was still waiting for consultation with a cardiologist and a plastic surgeon.
Patient no. 7: She underwent a rapid adrenocorticotropic hormone test and an anti-extractable nuclear antigen (ENA)/DNA test shortly after consultation with an endocrinologist and rheumatologist. Consequently, seronegative rheumatoid arthritis was confirmed.
Patient no. 8: She had no abnormal laboratory findings except insufficient vitamin D (22.43 ng/mL) and positive ANA. Magnetic resonance imaging (MRI) of the brain revealed no abnormal resonance imaging. The results of anti-ENA/DNA tests were normal. Furthermore, the patient lost curvature of the cervical spine with spondylosis on X-ray and was treated for possible benign headaches.
Patient no. 9: He was taking xanthine oxidoreductase regularly for gout and hyperuricemia. Severe swelling of the right knee with pain occurred 3 days after vaccination. The uric acid level was 2.4 mg/dL. Ultrasonography revealed a suprapatellar effusion without crystals. Synovial fluid analysis showed a pH of 7.4, white blood cell count of 273 cells/μL, and mucoid clot formation. The rheumatologist administered a triamcinolone injection for possible reactive arthritis, and the patient was still under observation for treatment response.
Patient no. 10: She was diagnosed with rheumatoid arthritis and was initiated on nonsteroidal anti-inflammatory drugs, methotrexate, hydroxychloroquine, and corticosteroids. The main symptoms, lower leg pain and swelling after vaccination, improved.
Patient no. 11: Most of the test results did not correlate with her symptoms. She was followed up at the cardiology outpatient department for possible variant angina.
Patient no. 12: Stomatitis occurred on the day of inoculation. When stomatitis was relieved with conservative treatment, she experienced a loss of taste and tingling on her tongue. The test results for Sjögren’s syndrome were negative. A steroid gargle was prescribed to manage ageusia, but the definite cause was not identified.
Patient no. 13: The antibody test results were normal, and the ultrasound test showed a mild increase in rheumatoid factor. We could not find a definite cause, but her right lower tibial pain and tenderness were severe enough to interfere with routine life. She was awaiting MRI results to rule out osteomyelitis.
Patient no. 14: Her main problem was anxiety with palpitation and breathing difficulty, although most of her medical test results, including complete blood count, electrolyte, creatine kinase-MB, troponin, gas analysis, echocardiography, electrocardiography, and chest radiography, were normal except for vitamin D levels (12.46 ng/mL). Her symptoms met the criteria for panic disorder with agoraphobia according to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Escitalopram, alprazolam, and propranolol were administered. Their response was good enough to relieve her symptoms, and she was referred to a psychiatrist.
Patient no. 15: She had no symptoms of bleeding tendency or arthralgia, although mildly abnormal laboratory findings were observed. Therefore, considering her history of panic disorder, a serotonin reuptake inhibitor was prescribed. She was followed up in the hospital in her hometown and treated for vestibule neuritis. One month later, the adverse reactions initially reported resolved.
Patient no. 16: The patient was previously diagnosed with hypothyroidism and Hashimoto’s thyroiditis. The patient complained of general weakness and fatigue. Her thyroid-stimulating hormone level was 0.185 μIU/mL; however, her results were otherwise normal. More specific history-taking revealed that she had taken Synthroid drugs with meals irregularly, which had been taught to intake regularly in a fasting state. Symptoms of hypothyroidism were suspected, and she was referred back to her primary care endocrinologist.
Patient no. 17: She complained of swelling, redness, and pain near the vaccination site. The patient was treated with oral antibiotics for a few days.
Patient no. 18: He sought consultation for chest wall and left shoulder pain following vaccination. He tested positive on the “Yergason,” and Empty Can tests on physical examination. The patient was evaluated for rotator cuff disease. Additionally, an increased bilirubin level was incidentally found, and the patient was diagnosed with Gilbert syndrome.
DISCUSSION
Many countries are currently attempting to overcome this pandemic. Vaccination is currently considered the only solution for preventing the spread of infection. However, adverse effects would naturally scare the public because of the excessive attention and expectations of new vaccines.
The United Kingdom began inoculation early, and a large-scale selfreported study involving 627,383 individuals was published between December 8 and March 10, 2021 [2]. Systemic effects after the first dose were more common in AstraZeneca than that in Pfizer. In individuals who received Pfizer, reports of systemic effects were more frequent after the second dose. Furthermore, significant differences in the ratio of reporting based on age and sex were observed between patients aged 55 years or younger and patients over the age of 55 years in terms of systemic and local effects for both AstraZeneca and Pfizer. Furthermore, women reported more adverse effects than men. A study of 515 individuals in Saudi Arabia found that fever and fatigue were more common after vaccination with AstraZeneca than Pfizer [3].
In the present study, significant differences in the frequency of certain symptoms among vaccine brands were not confirmed, although some symptoms were present in higher proportions in certain brands, such as a high ratio of skin lesions in AstraZeneca, chest pain in Pfizer, and cellulitis in Moderna. No other factors leading to adverse effects after vaccination were identified except for age. The accumulation of more data is needed to obtain more conclusive findings.
Additionally, the severity of symptoms, immunological medical history, and laboratory data, such as vitamin and antinuclear antibodies (ANA) level, were assessed, but no significant associations were found. Therefore, further research on ANA and vitamin D levels may provide useful information for patients who experience adverse effects.
In some systemic reviews, the immunomodulatory effects of nutrition on systemic lupus erythematosus were suggested in vitamin deficiency conditions and showed that supplementary therapy could help regulate disease activity [4,5].
Acute inflammation affects retinoid and carotenoid metabolism. Moreover, vitamin A deficiency and inflammation may interact, resulting in a significant decrease in plasma retinol levels [6]. Low plasma retinol levels are associated with an increased risk of infection or multiple morbidities [6].
Vitamin D helps maintain cellular homeostasis, which is influenced by cytokines in both innate and acquired immunity [7]. Many experimental studies have investigated the correlation between vitamin D levels and autoimmune diseases. In another review, vitamin D modulated the immune balance by inhibiting T helper (Th)1 cells and their anti-inflammatory effects [8]. Vitamin D regulates Th2 responses, increases regulatory T-cell activity, and suppresses Th17 cells [8]. A review of vitamins A and D suggested that their metabolites may have significant effects on Th1/Th2/Th17 cell differentiation and regulatory T cell induction [9].
High levels of homocysteine have been suspected to be related to some pathologies, such as atherosclerosis, cardiovascular disease, dementia, or cancer, although vitamin B supplement therapy is controversial. In one clinical study, the authors showed that high levels of homocysteine might be involved in the activity of Behçet’s disease regarding endothelial dysfunction caused by vascular inflammation [10].
Finally, a personal medical history or a state of inflammation could be the major factor behind various symptoms. As shown in the clinical courses of 13 out of 18 cases (except patient no. 3, 9, 11, 12, and 13), the possible causes for symptoms were revealed. In patients who were treated or suspected of having an immunologic disorder, more detailed history taking and evaluation are needed to elucidate the underlying causes. Furthermore, nutritional counseling may help patients such as patient no. 1 or no. 10 to be reassured and encouraged to be vaccinated with an additional dose.
Adverse events after the COVID-19 vaccination have also become a major issue. Unproven or exaggerated rumors only add confusion. Distorted facts may cause excessive anxiety or the inappropriate use of medical resources. Even if the severity of symptoms was similar, the ratio of patients visiting the outpatient department to those visiting the emergency department in this hospital approached approximately 1:10 in the last few months. This could increase the medical price and waiting period for other emergent patients. Therefore, an objective evaluation of the association between every symptom and vaccination needs to be conducted to normalize the routine of the entire medical system, which has since been disrupted by COVID-19. A careful approach is needed for elderly patients, as shown in Table 1, severe events to total adverse effects increased higher from the sixties. Additionally, general weakness with other abnormalities, such as swelling or joint pain, after vaccination in the elderly may suggest that nutritional or rheumatologic investigations should be conducted. Further studies are required to determine the relationship between nutrition and inflammation and what vaccines can trigger.
In conclusion, the range of adverse effects of available vaccines against COVID-19 is too broad to be defined. Moreover, the relationship between adverse effects and vaccination could not be established because of many other possible causes of adverse events. The patients varied widely in their symptoms and medical history, requiring advice from multidisciplinary teams. The case findings showed no evidence of acute heart disease or central nervous system damage, although many patients experienced chest pain, cardiopulmonary symptoms, and headaches. For instance, only two patients (patient no. 6 and no. 11) of the 12 patients with chest pain were referred to a cardiologist. Furthermore, close interviews revealed that one had a reasonable personalized history of his symptoms.
This study showed that taking detailed patient history is necessary to uncover possible underlying causes and to conduct a differential diagnosis. Further studies and the accumulation of additional data are needed.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.