Assessing Pulmonary Tuberculosis Using Bandim Tuberculosis and Karnofsky Performance Scale Scores with Serum Adenosine Deaminase Levels
Article information
Abstract
Background
Elevated pulmonary serum adenosine deaminase (ADA) levels signify lung tissue damage and severe tuberculosis (TB). Serum ADA assays can be used as an additional criterion for assessing TB treatment response and as a prognostic marker in patients with pulmonary TB. The Bandim TB and Karnofsky Performance Scale (KPS) scores were developed based on available clinical data and investigations to allow physicians to evaluate disease treatment and response. This study examined the use of a clinical scoring system (Bandim TB and KPS scores) in the context of serum ADA activity.
Methods
Forty adults (aged >18 years) diagnosed with pulmonary TB by Ziehl-Neelsen staining for acid-fast bacilli and/or cartridge-based nucleic acid amplification test were recruited. Standardized questionnaires were used to record Bandim TB and KPS scores. Serum ADA levels were estimated using a commercial kit.
Results
The Bandim TB score was positively associated (ρ=0.74, P≤0.001) and the KPS score was negatively associated (ρ=-0.69, P≤0.001) with serum ADA levels.
Conclusion
Subjective and objective clinical scores of pulmonary TB were strongly correlated with serum ADA levels. Knowledge of clinical scores corresponding to serum ADA levels could help physicians understand stage and progression of the disease which may aid in early detection and better management, and reduce disease transmission in a TB-endemic country.
INTRODUCTION
Tuberculosis (TB) is a major health problem caused by a group of closely related bacterial species called the Mycobacterium tuberculosis complex. As per World Health Organization (WHO) reports, approximately 7.1 million people worldwide were newly diagnosed with TB in 2019 [1]. TB is commonly diagnosed through smear microscopy, culture, and GeneXpert MTB/RIF tests, with culture being the gold standard [2]. However, conventional solid culture has a turnover time of up to 10–12 weeks, while smear microscopy has quality control issues and poor sensitivity [3]. Liquid culture techniques have been developed for early detection of mycobacterial growth, but this diagnostic test cannot yet control disease transmission as it has a 21-day turnaround time [4].
In recent years, various potential biomarkers for rapid diagnosis and monitoring of TB progression have been reported. Adenosine deaminase (ADA) is an enzyme present in T and B lymphocytes; its level increases during T-cell differentiation [5]. It plays a role in purine catabolism, catalyzing the deamination of adenosine to inosine and deoxyadenosine to deoxyinosine [6]. Serum ADA activity increases during pulmonary TB; therefore, ADA has high specificity and sensitivity for supporting TB diagnoses [7]. Elevated serum ADA levels in pulmonary samples signify lung tissue damage and severe disease. Hence, ADA measurement is a potential criterion for assessing TB treatment response, as well as a prognostic marker in patients with pulmonary TB [8]. Changes in serum ADA levels through treatment phases have previously been compared [6], and its possible utility in treatment monitoring has been assessed by correlating new pulmonary TB cases with patients with smear-positive TB experiencing sputum conversion [9].
Along with the previously mentioned TB detection methods, physicians require clinical scores to evaluate disease and treatment response. The Bandim TB and Karnofsky Performance Scale (KPS) scores were developed based on available clinical data and investigations [10].
The Bandim TB score was developed in Guinea-Bissau to make clinical decisions based on systematic evaluation of signs and symptoms, which can be challenging to monitor. The validity of this system has been evaluated in a cohort of patients with pulmonary TB in Bissau and Ethiopia [10]. This objective tool is based on five self-reported symptoms: cough, chest pain, dyspnea, hemoptysis, and night sweats, as well as six signs identified on examination: temperature >37°C, anemia, pulse >90 beats/min, positive findings at lung auscultation, body mass index (BMI) <18.0 kg/m2/<16.0 kg/m2, and mid-upper arm circumference (MUAC) <220 mm/<200 mm [10]. These cutoffs were chosen according to the previous literatures [11,12]. The cut-off for wasting was set at MUAC <220 mm and severe wasting at MUAC <200 mm [13]. The 11 clinical variables contribute one point each to the score, while a BMI of <16.0 kg/m2 and MUAC of <200 mm add an extra point each, adding up to a maximum possible score of 13. Based on this score, three severity classes (SCs) were determined: SC I, score 0–5; SC II, score 6–7; and SC III, score 8–13 points [12]. This score has advantages over the WHO standard in measuring TB outcomes [14].
KPS is a subjective rating tool to evaluate baseline performance and treatment outcomes and rates patients’ performance from 0%–100% according to their ability to perform various tasks. Patients are divided into three severity categories based on their ability to perform daily activities, ability to work, need for assistance, and presence of disease-related symptoms [10]. The KPS score is related to disease prognosis, and can therefore guide physicians in developing treatment strategies [15]. Assessing this score through a brief interview may allow for early diagnosis and better prognosis of TB.
Although KPS has been used as both a diagnostic and prognostic marker, serum ADA is not primarily used to diagnose pulmonary TB. Similarly, this study did not aim to evaluate serum ADA levels as a diagnostic or prognostic marker. Rather, it aimed to optimize a wellstudied clinical scoring system with serum ADA levels owing to their prior association with pulmonary TB. We evaluated associations between Bandim TB and KPS scores with serum ADA levels. This could help physicians improve patient management in pulmonary TB-endemic areas.
METHODS
This cross-sectional study was conducted from November 2019 to October 2021. Forty adults (aged >18 years) diagnosed with pulmonary TB by Ziehl–Neelsen (ZN) staining for acid-fast bacilli and/or cartridge-based nucleic acid amplification test (CB-NAAT) were recruited from the Medicine Department and Directly Observed Therapy Short-Course (DOTS) center of University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi. The inclusion criteria were treatment-naive cases and age >18 years. Patients with comorbid conditions or severe illnesses, including carcinoma, hepatic disease, or a known history of hematopoietic illnesses, were excluded. Figure 1 shows a flowchart of the study conduction process. Baseline ADA estimation and clinical scores were checked before starting antitubercular treatment. Standardized questionnaires were used to record Bandim TB (Table 1) and KPS (Table 2) scoring parameters.
ZN staining and CB-NAAT were performed in our laboratory as routine diagnostic methods, as per the Indian Revised National Tuberculosis Control Programme (RNTCP) guidelines, adapted from WHO guidelines [16].
1. Sputum Sample for Direct Microscopy
Direct smears were prepared from samples, and ZN staining was performed following RNTCP guidelines. M. tuberculosis appeared long, slender, beaded, and non-uniformly red or pink against a blue or green background. At least 100 oil immersion fields were examined for 10–15 minutes before giving a negative report.
2. Cartridge-Based Nucleic Acid Amplification Test
CB-NAAT was performed on sputum samples using the Xpert MTB/RIF assay (Cepheid Inc., Sunnyvale, CA, USA) at the DOTS center, Guru Teg Bahadur Hospital, Delhi, as per the manufacturer’s instructions. M. tuberculosis was identified when at least two of the five probes gave positive signals with a cycle threshold of ≤38 cycles.
3. Serum Adenosine Deaminase
Diagnostic kits (Audit Diagnostics, Cork, Ireland) were used to estimate serum ADA levels. Samples were tested using a fully automated chemistry analyzer (Rx Imola, Randox Lab, Bengaluru, India). For the estimation of serum ADA levels, 40 age- and sex-matched healthy controls were selected among patient attendants, hospital volunteers, or health care workers who had no medical complaints.
4. Ethical Clearance
Ethical clearance from the Institutional Ethics Committee of Human Research (IEC-HR) University College of Medical Sciences was obtained (IEC-HR/2019/41/68). Written informed consent was obtained from all patients before conducting the study. Study participation, including the right to withdraw at any time without explanation, was entirely voluntary.
RESULTS
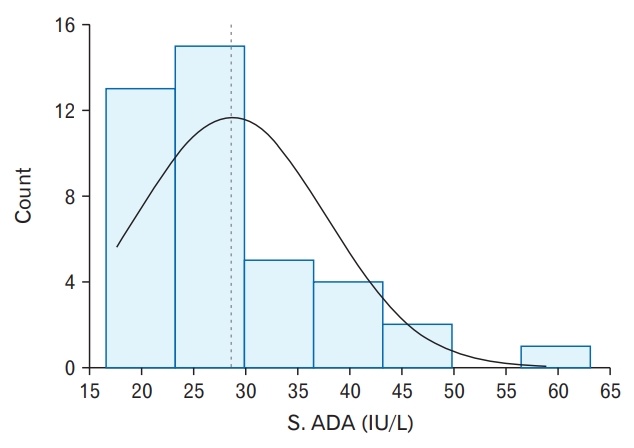
The patients in this study were aged 18–53 years. Table 3 depicts age and sex distributions of the study participants. The age group of 21–30 years accounted for the majority of cases (21 [52.50%]). There were 25 (62.5%) men and 15 (37.5%) women. Figure 2 depicts the distribution of serum ADA levels in the study population. Serum ADA (IU/L) levels were not normally distributed (Shapiro-Wilk Test: P≤0.001) and ranged from 17.61–58.92 IU/L in the study population, with mean (standard deviation, SD) and median levels being 28.60±9.08 IU/L and 26.70 IU/L, respectively. In the control group, mean±SD serum ADA level was 20.94±10.4 IU/L, with a range of 10.56–29.73 IU/L (P<0.0001).
1. Correlation between the Bandim Tuberculosis Score and Serum Adenosine Deaminase Levels
Figure 3 (scatter-plot diagram) depicts a strong positive correlation between the Bandim TB score and serum ADA levels. The Spearman correlation coefficient was 0.7 and P-value was <0.001.
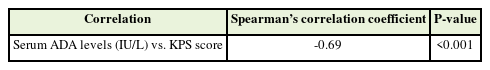
2. Correlation between the Karnofsky Performance Scale Score and Serum Adenosine Deaminase Levels
Table 4 shows the correlation between the KPS score and serum ADA levels. A strong, significantly negative correlation was found between the two variables (ρ=-0.69, P<0.001). For every 1-unit increase in serum ADA levels (IU/L), the KPS score decreased by 0.88 units. Conversely, for every 1-unit increase in the KPS score, serum ADA levels (IU/L) decreased by 0.53.
DISCUSSION
Despite improvements in diagnostic strategies, TB remains a serious public health concern; the inability to promptly diagnose the disease has rendered prevention strategies inadequate. Although the WHO has endorsed GeneXpert molecular testing technology for TB detection, its availability and cost restrictions pose problems [17,18]. We documented higher levels of serum ADA in the study group than in the control group (P<0.0001). This is consistent with previously reported findings that mean±SD serum ADA levels were 35.6±15.3 IU/L (range, 17–91 IU/L) in patients with smear-positive pulmonary TB and 19.0±7.1 IU/L (range, 9–32 IU/L) in controls (P<0.0001) [9]. A study by Lakshmi et al. [19] concluded that mean±SD serum ADA levels were higher in patients recently diagnosed with TB (33.52±15.22 IU/L) than in healthy controls (16.5±3.18 IU/L). Similarly, Bhandari et al. [20] reported that mean±SD serum ADA levels were 35.08±2.28 IU/L in patients with pulmonary TB, compared with 19.70±2.23 IU/L in controls. Another study showed that pre-treatment serum ADA values in patients with pulmonary TB ranged from 33.46–49.50 U/L, with mean±SD ADA levels of 40.22±0.79 U/L [21]. Various studies on patients with pulmonary TB have reported increased serum ADA levels in these patients, despite these measurements not generally being used as a diagnostic marker for TB [22]. Normal lymphocyte metabolism, partially regulated by the purine-salvage enzyme ADA, is required for effective cell-mediated immune response [23]. Activation of cell-mediated immunity in pulmonary TB may lead to increased serum ADA levels, and a decrease in serum ADA levels is observed concurrently with decrease in lymphocytes upon controlling TB infection. This makes serum ADA a suitable marker for treatment response [22,24].
We found a strong, significantly positive correlation (Spearman’s ρ=0.7) between serum ADA levels and the Bandim TB score (ρ=0.74, P≤0.001). Wejse et al. [14] concluded that the Bandim TB score was sensitive to changes during TB treatment and could be clinically useful in predicting mortality. Our study also showed a strong, significantly negative correlation (Spearman’s ρ=-0.7) between serum ADA levels and the KPS score. A higher Bandim TB score indicates poor prognosis, whereas a higher KPS score indicates good prognosis. A study conducted by Janols et al. [25] evaluated TB scores in patients with pulmonary TB during the intensive phase of treatment and reported a significant decline in scores after 2 weeks of treatment. Aunsborg et al. [26] reported a three-fold increase in TB coinfection detection after using TB scores in patients infected with human immunodeficiency virus. In another study conducted in Indonesia, multidrug-resistant-TB severity was assessed using modified Bandim scoring [27]. Though it was initially used to evaluate treatment response among patients receiving chemotherapeutic agents, limited studies have been conducted on the use of KPS score [15]. Rudolf et al. [10] evaluated reliability of the Bandim TB score compared with the KPS score in his study among patients with pulmonary TB and concluded that it exhibited a better performance than the KPS score.
To the best of our knowledge, no literature has correlated the Bandim TB and KPS scores with serum ADA levels in patients with pulmonary TB, despite these levels being significantly associated with pulmonary TB in various ways, as described earlier. In this study, we have strongly correlated subjective and objective clinical scores with serum ADA levels. This might be useful in rapid assessment of patients with TB, and thus help in early detection and better management of the disease. Knowledge of clinical scores corresponding to various serum ADA levels can help physicians understand TB prevalence, as well as reduce disease transmission in TB-endemic countries. Moreover, therapeutic responses to TB may be further improved by serial examination of serum ADA levels and correlation with clinical scoring parameters.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We acknowledge DOTS Center GTB for CB-NAAT.