Family Involvement to Stop the Conversion of Prediabetes to Diabetes
Article information
Abstract
Prediabetes is a condition associated with an increased risk of developing diabetes, in which blood glucose levels are high but not high enough to be diagnosed as diabetes. The rapid increase in the prevalence of prediabetes is a major global health challenge. The incidence of prediabetes has increased to pandemic levels and can lead to serious consequences. Unfortunately, nearly 90% of prediabetic individuals are unaware of their ailment. A quarter of prediabetic individuals develop type 2 diabetes mellitus (T2DM) within 3–5 years. Although prediabetes is a reversible condition, the prevention of diabetes has received little attention. It is essential for prediabetic individuals to implement new health-improvement techniques. Focusing on family systems is one strategy to promote health, which is determined by health patterns that are often taught, established, and adjusted within family contexts. For disease prevention, a family-based approach may be beneficial. Family support is essential for the metabolic control of the disease. This study aimed to show several strategies for involving the patient’s family members in preventing the conversion of prediabetes to T2DM and to emphasize that the patient’s family members are a valuable resource to reduce the incidence of diabetes.
INTRODUCTION
Prediabetes is a metabolic disorder in which blood glucose levels are elevated, but not high enough to be diagnosed as diabetes. The American Diabetes Association (ADA) and World Health Organization (WHO) use different criteria to define prediabetes based on blood glucose levels [1,2]. Prediabetes develops when the beta cells of the pancreas are unable to produce enough insulin to preserve blood glucose levels, when an individual’s body is unable to utilize insulin efficiently enough to reduce blood glucose levels, and when the beta cells in the pancreas fail to release sufficient insulin [3].
Prediabetes is identified based on two fundamental criteria established by the WHO: (1) fasting plasma glucose (FPG) levels between 110 mg/dL and 125 mg/dL referred to as impaired fasting glucose (IFG) and (2) impaired glucose tolerance (IGT), which is defined as 2-hour plasma glucose levels between 140 mg/dL and 200 mg/dL following the intake of 75 g of oral glucose load, or a combination of both, as determined with a 2-hour oral glucose tolerance test (OGTT). In contrast, the ADA describes prediabetes using three diagnostic criteria: (1) IGT with cutoff values of 140–200 mg/dL; (2) the cutoff value for IFG is lower (100–125 mg/dL), and (3) glycosylated hemoglobin (hemoglobin A1C [HbA1c]) levels ranging from 5.7% to 6.4% [1,2]. The lower threshold for IFG in the ADA criteria increases the number of patients diagnosed with IFG and IGT. In addition, these parameters lead to greater prevalence rates than those that conform to the WHO guidelines. IFG, which measures hepatic insulin resistance, is considered a better diabetes risk indicator than IGT, which assesses the insulin resistance of the skeletal muscle [4]. The ADA has also proposed testing for prediabetes using HbA1c levels. Many researchers agree that HbA1c is a reliable predictor of impaired glucose homeostasis because it represents stable blood glucose levels for several months and is not susceptible to daily fluctuations that can distort IGT and IFG measurements [5]. The ADA guidelines state that an abnormal measurement of any of these three indicators is sufficient to confirm prediabetes [6].
The diagnostic criteria suitable for diagnosing patients with prediabetes are disputed. The ADA recommendations are updated on a regular basis with the most up-to-date scientific evidence and may be more broadly applicable [7]. The outcomes of patients with prediabetes detected using each approach are highly heterogeneous and have little overlap [8].
Due to the challenges involved in the diagnosis of prediabetes, analyzing the relative prevalence of prediabetes from literature may be difficult [7]. However, the International Diabetes Federation has published details of current and future developments in prediabetic people aged 20 to 79 years based on IGT [9]. The global prevalence of IGT was reported to be 343 million (7.8%) in 2010, with rates ranging from 5.8% in Southeast Asia to 11.4% in North American and Caribbean countries. The prevalence of prediabetes is expected to increase to 471 million people worldwide by 2035 and 587 million by 2045 [9].
Diabetes affects 75 million individuals in Southeast Asia (8.3% of the adult population) and 138 million individuals (8.5% of the adult population) in the Western Pacific, placing a disproportionate burden on them. As a result of this upward trend, Asia is anticipated to account for more than 60% of the world’s diabetic population [10]. In addition, Asians have a diverse racial and genetic propensity to diabetes as well as a lower tolerance for environmental exposure [10]. In the Asian zone, a higher frequency of prediabetes and diabetes is observed among Indians (18.9% and 37.9%, respectively) and Malays (22.6% and 23.8%, respectively). Diabetes is more prevalent among females (21.9%) and city inhabitants (21.5%) [11].
WHY SHOULD WE BE CONCERNED ABOUT PREDIABETES?
The Center for Disease Control and Prevention reports that nearly 90% of prediabetic patients are unaware of their condition [12]. Prediabetes can progress to type 2 diabetes mellitus (T2DM) in as little as 5 years in up to 70% of patients with the condition [13]. Within 3–5 years, approximately 25% of people with prediabetes develop overt T2DM and up to 70% of people will develop overt diabetes within their lifetime [12,13].
The rate at which people progress from prediabetes to diabetes varies depending on the population and the standards used to define prediabetes [14,15]. In the Diabetes Prevention Program (DPP) outcome analysis, the prevalence of diabetes in the control group was reported to be 11% [16]. The incidence of diabetes was reported to be >90% in patients with IGT, characterized by recurrent OGTT in controls over a 20-year period in the China Da Qing Diabetes Prevention Study. Individuals with both IFG and IGT have a higher chance of developing diabetes than those with IFG or IGT alone [14,17].
Prediabetes has reached epidemic proportions and can cause severe complications as the disease progresses. Several disorders are commonly associated with diabetes, such as nephropathy, neuropathy, diabetic retinopathy, and macrovascular problems, and are more likely to occur in people with prediabetes [13]. Long-term diabetes, as a chronic disease, leads to a reduction in the quality of life and a considerable increase in health care expenses [9]. To stop the rising incidence of diabetes, awareness of prediabetes and action become crucial.
Diabetes prevention has not been implemented, even by healthcare providers who want to improve their health services. A study on prediabetes knowledge, attitudes, and practice found limited prediabetes diagnosis. Further, patients who tested positive for prediabetes were not referred to the active DPP [18].
The emphasis on diabetes treatment appears to assume that any focus on diabetes must begin with a patient diagnosed with the disease. However, this view ignores the importance of diabetes prevention in the provision of high-quality care. It is critical to effectively treat patients with diabetes, but diabetes prevention is essential to close the gap with diabetes management in terms of clinical priorities in primary care [19].
Prediabetes is a treatable condition that increases the risk of developing diabetes. Improved recognition and risk stratification of people with prediabetes allows clinicians to gain a better understanding of the most effective therapies to reduce the number of patients under their care who develop diabetes. If they do not receive an intervention, up to 37% of patients with prediabetes will develop diabetes within 4 years [20].
Although prediabetes is generally acknowledged and endorsed by major global guidelines on diabetes mellitus (DM), practitioners and health professionals still lack a good understanding and knowledge of the issue [21]. Consequently, identifying and treating people with prediabetes is crucial in our attempts to improve access to healthcare, decrease the disease burden, and protect those at risk [20].
MANAGEMENT OF PREDIABETES
The main goal of prediabetes treatment is to maintain normal glucose levels while averting or postponing the onset of diabetes and associated microvascular complications [22,23]. Prediabetes treatment can be divided into three categories based on the treatment used: lifestyle changes, pharmacological interventions, and surgical interventions [24]. High-intensity lifestyle interventions and pharmacological interventions (metformin) are the two most widely recognized and used interventions for diabetes prevention [25,26]. All related literature discuss the debate regarding optimal intervention strategies for preventing diabetes [27]. Some studies recommend only high-intensity lifestyle interventions [28], which may not be appropriate for all patients [24,29].
1. Lifestyle Intervention
Lifestyle modification is the first-line intervention for people with prediabetes. It includes dietary changes, exercise, and avoiding smoking and alcohol [30]. The two most prominent modifiable risk factors for the development of diabetes are obesity and physical activity, and lifestyle interventions should focus on them [24]. Individuals should be encouraged to reduce their body weight by 5%–7% by restricting caloriedense foods and engaging in 150 minutes or more of moderate-intensity physical activity each week [31].
The DPP trial is the first to compare lifestyle against pharmacotherapy for weight loss and diabetes risk reduction in a US population and found that the behavioral intervention group lost more weight than the metformin and placebo groups. Nearly half of the participants in the rigorous lifestyle change group lost at least 7% of their initial weight and preserved it [32]. The DPP study found that dietary changes resulted in a greater decline in the incidence of diabetes than metformin use (58% versus 31%) [33].
2. Pharmacological Intervention
The second line of treatment is pharmacological intervention [30]. Currently, the Food and Drug Administration has not granted approval for any pharmacotherapy treatment options to control prediabetes or prevent disease progression to diabetes [32,33]. Pharmacotherapy is recommended for patients at high risk of impaired glucose regulation. This is also true for patients who are unwilling or unable to change their lifestyle or have adverse reactions to lifestyle changes [34].
Despite reported side effects such as gastrointestinal side effects and the ability to lower vitamin B12 levels, the ADA has given metformin an “A” rating for diabetes prevention [25]. Patients with prediabetes who have risk factors such as age <60 years, body mass index (BMI) >35 kg/m2, a history of gestational DM, and/or those with elevated HbA1C amidst lifestyle modifications are recommended to take metformin, in conformity with the ADA [33].
In the DPP report, metformin alone was associated with a 31% decline in the incidence of diabetes when compared to placebo. The risk of T2DM in the metformin group was 18% lower than that in the placebo group after 10 years of follow-up [16]. In the US DPP trial, metformin was reported to be less effective than lifestyle modifications; however, in the Indian DPP study, it was revealed to be equally effective as lifestyle modifications [32,35].
Other pharmacological agents such as thiazolidinediones (pioglitazone, rosiglitazone, and dapagliflozin), glucosidase inhibitors (acarbose), and glucagon-like peptide 1 analogs (exenatide) have shown promise in delaying the development of diabetes [36]. In obese and overweight patients, no single drug is the most effective in terms of weight loss and diabetes prevention. Patient interests, comorbidities, and side-effect profiles should all be considered before starting pharmacotherapy [37].
3. Surgical Intervention
Bariatric surgery requires several procedures to reduce caloric intake by inducing a malabsorption state, restrictive state, or a mixture of both [36]. This intervention has shown tremendous potential to reverse the development of diabetes, especially in obese individuals with prediabetes [30]. Long-term weight loss related to bariatric surgery results in a significant 2-year and 10-year reduction in the incidence of diabetes in morbidly obese individuals [38,39]. Bariatric surgery resulted in longterm weight reduction (23.4% after 2 years and 16.1% after 10 years) and a 75% decrease in the relative risk of diabetes in Swedish obese subjects compared to controls [38].
THE IMPORTANCE OF FAMILY APPROACH IN DIABETES PREVENTION
Prediabetes and diabetes are ongoing health challenges, and new health-improvement techniques must be enforced. Since health patterns are usually taught, established, maintained, and modified in family settings, focusing on family systems is one way to promote the well-being of individuals, families, and communities [40].
The Institute for Patient and Family-Centered Care defines family members as two or more persons who are biologically, lawfully, or psychologically related in a certain way [41]. Consequently, family members may be those from the core family, extended family, or friends [42].
Most patients receive primary care, and healthcare providers are responsible for examining health issues and treating the majority of risk factors for highly ubiquitous and aggravating diseases in numerous families and relatives. Therefore, using a primary care approach may be very beneficial [40].
Most prevention strategies focus on specific diseases, and underestimate the possible consequences of comparable pathophysiological pathways in other health disorders. The majority of research on behavioral and preventative measures is conducted with patients, and results are often measured in sick people, ignoring the impact of care on others who may also benefit. Consequently, a broader approach that focuses on the family rather than the individual may be particularly beneficial for disease prevention and health promotion [40].
Individuals learn and maintain habits practiced by their families as part of their culture, collectively influencing the creation of healthy and unhealthy behaviors. Families also encourage and reinforce good health among their members by using various forms of social support (emotional, instrumental, informative, and judgmental) [43]. As a result, families with positive relationship styles have healthier attitudes, whereas those with unfavorable relationship styles have inferior health results [44].
Several studies and meta-analyses have been conducted to determine the efficacy of family-centered approaches for the treatment and prevention of physical illnesses [45-49]. They all agree that this method is superior to traditional medicine. However, research on family care is limited, and much of it is focused on treating rather than preventing certain health problems (such as diabetes, arthritis, obesity, asthma, coronary heart disease, and cancer) [40].
The role of family interactions has been overlooked in most previous management intervention studies on prediabetes. The patients’ first interactions with their families may have various effects. Inadequate or absent family support can contribute to metabolic dysregulation by restricting effective disease management and care. Family support can be critical in regulating disease metabolism by fostering an environment that is less stressful and more conducive to patient adherence, and can help family members lead healthy lives [50].
Research on family involvement in diabetes prevention is rare. Vargas-Ortiz et al. [50] in 2020 conducted a randomized clinical trial comparing the effects of family interventions versus individual interventions on glucose metabolism, insulin resistance, β-cell function in pancreas, cardiovascular risk indicators, and familial metabolic risk. The patients and family members in this study received individualized diet and dietary therapy. Every 6 months, an interdisciplinary network of nutritionists, general practitioners, physical activity specialists, psychologists, and endocrinologists planned and managed group sessions lasting approximately 1 hour, covering topics related to diet, physical exercise, and coping with stress. After 12 months of follow-up, the study found that family interventions improved blood glucose, insulin sensitivity, lipid panel, and metabolic risks more effectively than individual interventions, whereas individual interventions increased the risk [50].
Adults with prediabetes and/or other family members, including children with risk factors, such as obesity, may benefit from family-based diabetes prevention strategies. They can participate in various activities, such as nutrition education through games that promote healthier eating habits, joint exercise sessions, and lifestyle modifications [51].
Involving the family in preventing the conversion of prediabetes to diabetes should be considered if patients have healthy or dysfunctional family features. Evidence suggests that functional families are associated with some of the main predictors of progression in diabetes prevention programs. Children from functional families have been shown to have greater weight loss, medication adherence, and healthier diets. However, the impact of family-based approaches on the prevention of obesity and diabetes has not yet been thoroughly investigated. Family functional measurements can use the McMaster model, Circumplex model, Family Environment Scale, APGAR (Appearance, Pulse, Grimace, Activity, and Respiration) score, or Family Assessment Measures [52-56].
It should be noted that functional families can provide two possible conditions when it comes to diabetes prevention programs, particularly weight loss. First, inadequate family functioning is associated with a higher BMI and a decreased ability to lose weight. Second, unexpected outcomes for functional families could be associated with a higher BMI and a slower rate of weight loss. Questions concerning family relationships, diet, culture, and obesity remain unanswered [51].
PROPOSED STRATEGIES TO INVOLVE FAMILY IN STOPPING THE PROGRESSION OF PREDIABETES
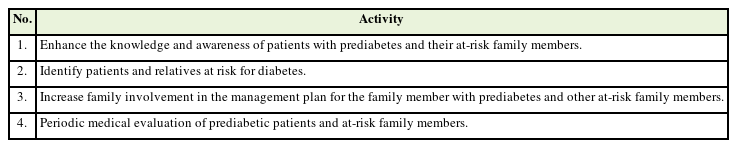
It is important to address the involvement of relatives in managing prediabetes so that family involvement in preventing diabetes is a priority in primary clinical care. In general, some measures to stop the progression of prediabetes are similar to those for diabetes management, with one difference being the prevention target. Table 1 shows the proposed strategies for the prevention of diabetes through family involvement.
1. Increase Knowledge and Awareness of Prediabetic Patients and Their Family Members at Risk
Clearly, it is important to know the right way to deliver the messages to the family. Patients should be taught about increased familial risk and the efficacy of lifestyle changes in lowering the risk of diabetes to improve the impact of family-based treatments [57].
Families may have additional concerns regarding the health of their relatives because of the information provided. In fact, some family members may have concerns regarding their future health. Emphasizing the importance of attention to patients may lead to more family discussions regarding the risk of diabetes [58]. It is well understood that some fear is required to elicit protective behaviors. However, if an individual does not believe that he or she is capable of taking risk-reduction measures, instilling fear can be counterproductive [59]. Consequently, emphasizing the possibility of diabetes prevention is critical at this phase, along with an understanding of how, when, and whom to advise [57].
People who were given additional information regarding their family’s diabetes risk reported greater knowledge concerning the risk and lifestyle changes to reduce the risk, as well as healthier eating habits, than those who were not informed about their family’s risk, according to a cross-sectional controlled trial and other studies [60,61].
Current prediabetes education programs typically concentrate on improving self-esteem and self-care skills, as well as improving diet, physical activity, weight management, and regular medical tests [62,63]. Because people with prediabetes and their relatives have no obvious symptoms or signs and sometimes do not recognize their disease, education and communication are important [64,65].
2. Identifying Patients and Family Members at Risk of Diabetes
Screening for risk factors, calculation of the magnitude of risk, and risk stratification are necessary for patients and their families. Prediabetes is part of a cascade model that includes ways to classify patients with prediabetes, relate to and keep them in care, and achieve treatment targets [66].
Providers are recommended to screen for prediabetes and T2DM and use risk assessment tools such as the ADA, Finnish Diabetes Risk Score (FINDRISC), or Ausdrisk risk test to determine if a diagnostic test is needed. Both prediabetes and T2DM matched the criteria for disorders that must be recognized early. These disorders are prevalent and have significant clinical and public health consequences [25].
Adults patients or families that are obese or overweight (BMI of 25 kg/m2 or 23 kg/m2 for Asian Americans) and have any of the risk factors listed below are assessed informally: diabetes in first-degree relatives, people from tall families, racial or ethnic risk (e.g., Pacific Islander, Latino, African American, Asian American, and Native American), lack of physical exercise, hypertension (140/90 mm Hg or currently on treatment), high-density lipoprotein cholesterol levels <35 mg/dL (0.90 mmol/L), and/or triglyceride levels >250 mg/dL (2.82 mmol/L), history of cardiovascular disease (CVD), women with polycystic ovary syndrome, and other medical conditions (such as severe obesity and acanthosis nigricans). It is recommended that these patients be tested further using laboratory tests for blood analysis [25].
A diabetes risk assessment tool was used to categorize individuals at risk of developing diabetes. To calculate a risk score, this tool usually consists of a series of questions regarding diabetes risk factors combined with simple anthropometric index measurements. Factors gleaned through intrusion techniques or laboratory-derived parameters have also been used [67]. There are more than 145 models of risk assessment tools currently available, and seven are suggested for everyday clinical practice owing to their strong adaptation capability, namely Germany-ARIC, Australia-Ausdrisk, UK-Cambridge risk score, Finland-FINDRISC, USA-Framingham ancestry, USA-San Antonio risk score, and UK-QDScore [68].
The ADA recommends that everyone undergo tests at the age of 45 years. Individuals who are overweight/obese and have one or more risk factors for diabetes should consider screening for prediabetes and the risk of developing diabetes in asymptomatic individuals. Prediabetes can be detected using HbA1C, FPG, or 2-hour plasma glucose levels after a 75-g OGTT. In addition, the ADA suggests that children and teenagers who are overweight or obese and have two or more risk factors for diabetes should undergo prediabetes testing [6].
3. Increasing Family Engagement in the Management Plan for the Family Member Who Has Prediabetes
Family support, as noted earlier, is critical in overcoming the task of persuading patients to strengthen and sustain their lifestyle improvements. Family plays an important role in regulating the patient’s metabolism by offering an appropriate atmosphere and increasing patient compliance. Patients with prediabetes and their families will face a lifestyle change in this situation [50]. One of the relatively new concepts of diabetes prevention is to use the patient as a family health educator. Family members of patients with prediabetes should be engaged and encouraged by health care providers to provide support in the form of knowledge, emotional support, behavioral support, attendance at educational sessions, and the ability to be peer leaders and problem solvers. Promoting family communication may be a viable strategy for preventing the progression of prediabetes to diabetes. Responses to these initiatives may vary, and sociodemographic factors may play a role [57].
In clinical practice, healthcare providers should initiate the non-pharmacological management of patients with prediabetes and their families at risk and provide detailed guidance and targets, as suggested by the ADA. Specific weight loss goals, specific nutritional therapy, and a minimum of 150 minutes of moderate physical activity per week are some of the strategies. Furthermore, non-pharmacological management of prediabetes should be the first-line treatment for individuals at a low risk of developing diabetes [69].
4. Periodic Medical Assessment of Prediabetic Patients and Family Members at Risk
Every prediabetic patient and their family who have been identified as having a high risk of acquiring diabetes is encouraged to visit the clinic regularly. Even after controlling for patient history and lifestyle, clinic visits after the diagnosis of prediabetes were associated with lower diabetes risk factors (such as BMI). These results highlight the importance of clinical follow-up for individuals diagnosed with prediabetes during the screening process [70].
To emphasize the ADA criteria and accomplish goals, healthcare professionals should seek to recreate comprehensive educational and training treatments for patients, which entail providing personalized advice for each patient, nutrition, loss of weight, physical exercise targets, and continuous monitoring. Study results indicate that close monitoring contributes to a successful outcome [69].
Patients with prediabetes should undergo an FPG and/or OGTT at least once a year to determine their glycemic status. Annual FPG and A1C assessments, and thus a 2-hour OGTT, should be performed in individuals with suspected disease progression [22,23]. Excessive weight gain and CVD risk factors (especially high blood pressure and/or dyslipidemia) should be treated and monitored regularly [71]. Patients with prediabetes should have their blood pressure monitored and tested for microalbuminuria at least once a year [23].
CONCLUSION
Families contribute substantially in regulating the metabolism of family members with prediabetes. Families can recognize and prevent T2DM by understanding the risk factors and helping with lifestyle modifications. Several innovative family-based interventions should be implemented to prevent the development of prediabetes to diabetes. Family engagement in the treatment of prediabetic family members is essential and can be part of a successful diabetes prevention strategy. The authors found that including family members in diabetes prevention strategies, to prevent or delay progression of prediabetes, was under-researched and rarely used to initiate or encourage behavioral change. Future research should explore innovative ways to prevent the development of diabetes in people with prediabetes by placing the patient’s family at the same level as the patient and incorporating them into diabetes prevention programs as part of an overall approach. In addition, family is one of the available resources that can assist diabetes prevention programs accomplish their objectives.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta.