Low Serum Creatinine as Well as High Serum Creatinine Is Associated with Prognosis of Patients with Cancer in End-of-Life
Article information
Abstract
Background
The prognosis of end-of-life patients is challenging, and clinicians have attempted to predict survival more accurately. High serum creatinine (sCr) levels are associated with lower survival rates in patients with various cancers; however, low sCr levels are commonly expected in patients with terminal cancer because of muscle wasting and malnutrition. Therefore, we investigated the prevalence of low and high sCr levels and their association with survival duration in patients with terminal cancer in a palliative care unit.
Methods
We analyzed the medical records of 280 patients admitted to a palliative care unit. Patients were divided into low (<0.5 mg/dL), normal (0.5–1.2 mg/dL), and high (>1.2 mg/dL) sCr groups. Kaplan-Meier survival curves using sCr levels were plotted and compared using the log-rank test. Using stepwise selection, a multivariable Cox proportional hazards model was used to identify the significant prognostic factors.
Results
The median survival durations in the high-, low-, and normal-sCr groups were 9.57 days, 22.26 days, and 27.51 days, respectively. Multivariable Cox proportional hazard model identified that males (hazard ratio [HR], 1.81; 95% confidence interval [CI], 1.16–2.85), poor performance status (HR, 3.43; 95% CI, 1.12–10.54), total parenteral nutrition use (HR, 1.84; 95% CI, 1.09–3.1), high sCr (HR, 2.74; 95% CI, 1.52–4.94), and low sCr (HR, 1.22; 95% CI, 1.07–1.43) were significantly associated with a shorter survival time.
Conclusion
Low and high serum creatinine levels were significantly associated with poor survival in patients with cancer at the end-of-life stage. Therefore, readily available and simple biomarkers may help plan advanced care in palliative care settings.
INTRODUCTION
Accurate prognosis is important for patients and caregivers during end-of-life care to ensure that the patient’s remaining time is meaningful and for physicians to establish appropriate care plans [1]. However, predicting the survival time of patients with cancer at the end of life is challenging because of their diverse disease courses and various physiological characteristics. Therefore, predicting the prognosis is challenging for clinicians caring for end-of-life patients with cancer.
Clinicians have attempted to estimate the survival time of patients using validated scales, such as the Palliative Prognostic Score [2] and the Objective Prognostic Score [3], by integrating subjective and objective findings. Subjective findings include the clinician’s prediction of survival and symptoms, such as dyspnea, anorexia, and delirium [4-6]. Objective findings include various biological parameters [7], such as prealbumin or cortisol levels; however, sometimes, these have little clinical significance in patients with terminal cancer [8]. Thus, factors predicting the exact time of the active phase of death in patients with terminal cancer remain ambiguous. Furthermore, in palliative care settings, where care is focused on patient comfort, only basic blood tests such as complete blood count and renal and liver function tests are performed to assess the patient’s status, and additional tests are required depending on the patient’s condition. Therefore, a universal, readily available, and cost-effective clinical parameter is necessary to predict the survival time of end-of-life patients with cancer regardless of age, sex, or cancer type.
Creatinine (Cr) is an amino acid compound derived from creatine and creatine phosphate [9]. Serum Cr (sCr) level is generally used as a standard biomarker to evaluate renal function [10]. Several studies have suggested that high sCr levels are associated with poor prognosis in patients with cancer owing to their association with renal dysfunction and cancer progression [11]. In patients with terminal cancer, renal function can decline for various reasons, such as multiple organ failure, dehydration, and nephrotoxicity due to cancer treatment, and it is assumed that the deterioration of renal function is related to the prognosis. However, low sCr levels are associated with poor survival in patients with terminal cancer, possibly because of decreased muscle mass due to poor oral intake and cachexia. To date, physicians have often focused on high sCr levels, and there is insufficient information regarding the prevalence and prognostic value of low sCr levels in patients with terminal cancer. Therefore, we aimed to determine the prevalence of abnormal sCr levels in patients with terminal cancer and its prognostic value in a palliative care setting.
METHODS
1. Participants
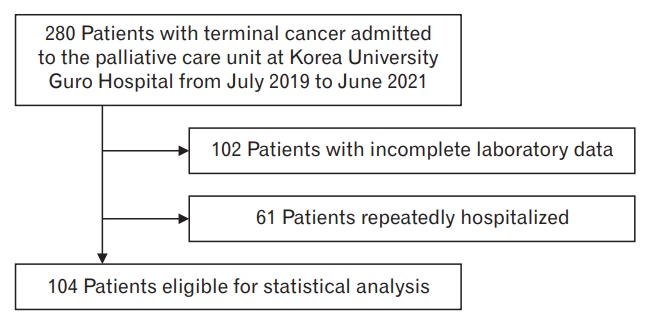
This was a retrospective study. We reviewed the medical records of 280 patients with terminal cancer admitted to the palliative care unit of Korea University Guro Hospital between July 2019 and June 2021. Among the 280 patients, those with incomplete routine laboratory data on hemoglobin (Hb) levels, white blood cell (WBC) counts, albumin, total bilirubin, C-reactive protein (CRP), magnesium (Mg), calcium (Ca), and creatinine levels were excluded (N=102). Patients who were repeatedly hospitalized and discharged within the study period were considered single cases based on their last hospitalization (N=61). Finally, 104 patients were included in the study (Figure 1). Patients with unavailable survival data due to discharge were excluded (N=13). Ethical approval for this study was obtained from the Institutional Review Board of the Korea University Guro Hospital (registration no., K2021-2502-001).
2. Data Collection
Patient demographics and clinical information regarding sex, age, primary cancer site, performance status, total parenteral nutrition (TPN) use, pain intensity, admission and transfer-in date, discharge date, date of death, and laboratory findings were collected from electronic medical records. Survival time was defined as the time from the date of admission to the date of death or censoring. The primary cancer sites were categorized according to the International Classification of Disease-10. Performance status was assessed using the Eastern Cooperative Oncology Group Performance Scale (ECOG-PS), and an ECOG score of 4 was considered to represent poor performance status [12]. Pain intensity was measured using the Numerical Pain Rating Scale (NRS) [13]. Patients were evaluated by physicians or trained palliative care nurses based on their ECOG-PS and NRS scores at the time of admission or transfer. In our palliative unit, routine laboratory examinations were performed within three days of admission, and additional tests were performed according to changes in the patients’ conditions. The results of the first test were analyzed if laboratory tests were repeated within 3 days of admission.
Patients were divided into low (<0.5 mg/dL), normal (0.5–1.2 mg/dL), and high (>1.2 mg/dL) sCr groups (sCr range, 0.5–1.2 mg/dL) [14]. The Hb, WBC count, albumin, total bilirubin, CRP, Mg, and Ca levels were divided and analyzed according to the in-hospital standard cut-off levels.
3. Statistical Analysis
Descriptive data are expressed as numbers (%) for categorical variables, which were measured using the chi-square test. The Kaplan-Meier method was used to compute the median survival time and 95% confidence intervals (CIs) for each group. Kaplan-Meier survival curves according to the sCr groups were plotted and compared using the log-rank test. Univariate and multivariate Cox proportional hazard models using stepwise selection were used to identify significant prognostic factors. Hazard ratios (HR) and 95% CIs were calculated. The level of statistical significance was P<0.05, except for the univariate Cox hazards model (P<0.15). All the statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
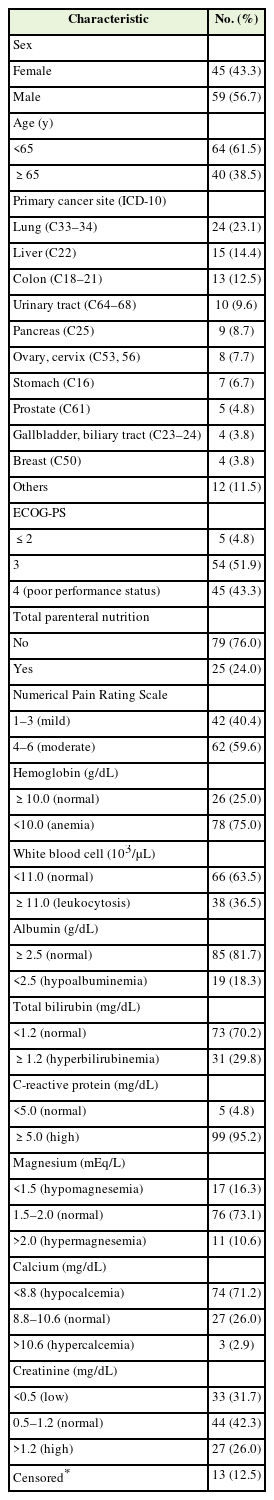
The demographics and clinical characteristics of the patients based on their sCr levels are presented in Table 1. Among the 104 patients, 56.7% (N=59) were men, and 38.5% (N=40) were aged ≥65 years. The most common primary cancer site was the lungs (N=24, 23.1%), and 43.3% (N=45) of patients had a poor performance status. The prevalence rates of low and high sCr levels were 31.7% and 27.0%, respectively.
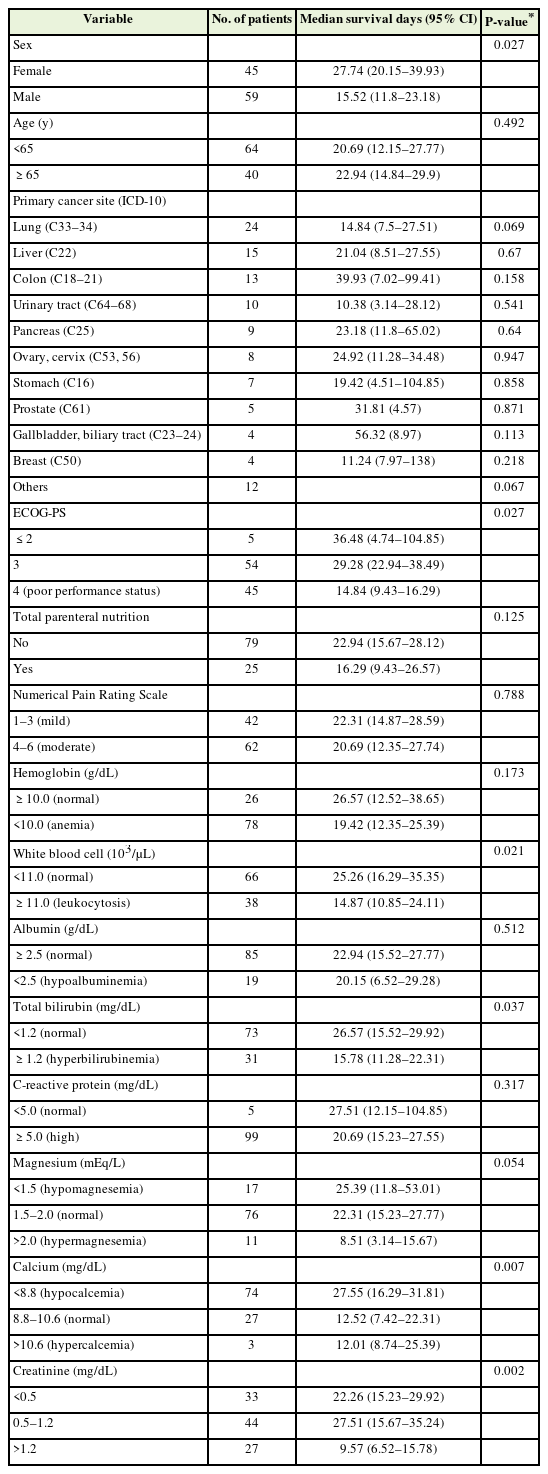
Table 2 shows the median survival time in relation to patient characteristics. Male sex, poor performance status, leukocytosis, hyperbilirubinemia, hypercalcemia, and high and low sCr levels were significantly associated with a shorter median survival time.
Figure 2 presents the Kaplan-Meier curves of the three groups categorized according to their Cr levels. The median survival time was shorter in the high sCr group (median survival time, 9.57 days; 95% CI, 6.52–15.78 days) compared to the low sCr group (median survival time, 22.26 days; 95% CI, 15.23–29.92 days) and normal sCr group (median survival time, 27.51 days; 95% CI, 15.67–35.24 days) (P=0.002, log-rank test).
The independent prognostic factors analyzed using multivariable Cox proportional hazard models are shown in Table 3. A multivariable Cox hazard model using stepwise selection identified that factors such as male sex (HR, 1.81; 95% CI, 1.16–2.85; P=0.010), poor performance status (HR, 3.43; 95% CI, 1.12–10.54; P=0.019), use of TPN (HR, 1.84; 95% CI, 1.09–3.1; P=0.023), and high sCr (HR, 2.74; 95% CI, 1.52–4.94; P=0.003) and low sCr levels (HR, 1.22; 95% CI, 1.07–1.43; P=0.003) were significantly associated with poor prognosis.
DISCUSSION
We found that more than half of the patients with terminal cancer had abnormal sCr levels, and low and high sCr levels were significantly associated with survival, regardless of the cancer type. Considering that sCr is a renal impairment marker, low sCr levels may not draw as much attention as high sCr levels, and there are few studies on the association between low sCr levels and overall survival. Both low and high sCr levels were significantly associated with lower overall survival in patients with colorectal cancer [15]; a U-shaped distribution was found for both low and high sCr levels at admission, and these were associated with increased in-hospital mortality [16].
Both increased renal clearance and decreased Cr production, leading to low sCr levels [17]. Augmented renal clearance is common among patients who are critically ill. Additionally, Cr is a product of muscle catabolism, and sCr levels are influenced by muscles [18]; advanced age and malnutrition are probable factors for decreased Cr production [17]. Patients with terminal cancer often experience cachexia and anorexia, which result in the loss of both skeletal muscle and fat [19]. Thus, low sCr levels represent the general condition of patients and are significantly associated with the survival of patients with terminal cancer.
Previous studies have noted that a high sCr level is a significant prognostic factor in patients with various types and stages of cancer [11]. The relationship between sCr and the remaining life expectancy of patients with terminal cancer can be explained by several factors. Decreased renal function may be the primary contributor to high sCr levels in patients with cancer. Furthermore, renal impairment is common in patients with cancer due to multiple organ failure, postoperative complications, or chemotoxicity. Cr metabolism is linked to cancer progression and metastasis through several mechanisms. Cr is the end product of creatine and creatine phosphate metabolism and is utilized as an essential energy source [9]. Cr is transported into the muscles and phosphorylated by creatine kinase (CK). Previous studies have shown that CK expression is upregulated during cancer progression [20]. Cancer cells use creatine as a readily available energy source; thus, the end product of creatine metabolism increases [21]. High sCr levels in patients with terminal cancer are related to cancer progression and are considered prognostic factors.
In addition to Cr level, several other factors are associated with survival in patients with terminal cancer. We found that males were associated with a shorter survival time. A different population-based study reported a survival disadvantage for males with 11 different cancers, including head and neck, esophageal, colorectal, pancreatic, lung, bone, melanoma, mesothelioma, kidney, thyroid, and non-Hodgkin lymphoma [22]. Although explanations related to sex hormones and health-promoting behaviors have been suggested [23], the exact cause of sex discrepancies in patients with terminal cancer remains unknown. We also found that TPN was associated with poor survival rates. This can be explained by the usual application of TPN in patients with insufficient oral intake, although the benefits of TPN in end-of-life cancer patients remain controversial [24].
Generally, hypercalcemia is related to the prognosis of patients with cancer because of the systemic secretion of parathyroid hormone-related proteins in various cancers and bone metastasis [25,26]. However, no such association was found in our study, probably because of the low prevalence of hypercalcemia. Hypocalcemia is common in patients with cancer, which is consistent with our study [27]. In a previous study, hypocalcemia was more strongly associated with better prognoses than normal serum Ca levels; however, this association was not significant in multivariate analysis [28]. The total Ca level is affected by albumin; thus, it is necessary to measure the ionized Ca levels accurately. Ionized Ca was not routinely measured in our palliative care unit; thus, we could not assess the association between hypocalcemia and a good prognosis.
This study had several limitations, mainly owing to its retrospective design. It was limited in terms of its application in patients with terminal cancers, who usually received limited tests to avoid discomfort. First, some confounders, such as body mass index (BMI), could not be analyzed. Moreover, the weight and height of patients are rarely measured in palliative care units because of their poor condition or because physicians prefer to avoid unnecessary discomfort. Additionally, measured body weight values may not be accurate because of the presence of ascites or lymphedema, and BMI values do not significantly influence survival times [29]. Second, as mentioned above, more significant laboratory findings, such as ionized Ca levels, could not be included in the analysis. Third, we did not exclude patients with renal cancer or chronic kidney disease, which may have affected the sCr data. Furthermore, this was a single-center study that did not include general patients with terminal cancer. Therefore, multicenter studies with larger sample sizes are required. Nonetheless, to the best of our knowledge, this study is the first to identify the prognostic value of low sCr levels in end-of-life patients with cancer. We also confirmed the association between relatively simple blood test parameters and survival time in palliative care settings. Further studies are required to explore the validity and reliability of low sCr levels as an independent prognostic factor.
We found that low and high sCr levels were significantly associated with poor survival in end-of-life patients with cancer. A readily available and simple clinical parameter may help predict the prognosis and make advanced care plans in palliative care settings.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the Korea University grant (grant number: K2209881).
Acknowledgements
The authors sincerely thank Korea University Guro Hospital for providing the data.