Evidence-Based Guideline for the Treatment of Smoking Cessation Provided by the National Health Insurance Service in Korea
Article information
Abstract
Although major countries, such as South Korea, have developed and disseminated national smoking cessation guidelines, these efforts have been limited to developing individual societies or specialized institution-based recommendations. Therefore, evidence-based clinical guidelines are essential for developing smoking cessation interventions and promoting effective smoking cessation treatments. This guideline targets frontline clinical practitioners involved in a smoking cessation treatment support program implemented in 2015 with the support of the National Health Insurance Service. The Guideline Development Group of 10 multidisciplinary smoking cessation experts employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-ADOLOPMENT approach to review recent domestic and international research and guidelines and to determine evidence levels using the GRADE methodology. The guideline panel formulated six strong recommendations and one conditional recommendation regarding pharmacotherapy choices among general and special populations (mental disorders and chronic obstructive lung disease [COPD]). Strong recommendations favor varenicline rather than a nicotine patch or bupropion, using varenicline even if they are not ready to quit, using extended pharmacotherapy (>12 weeks) rather than standard treatment (8–12 weeks), or using pharmacotherapy for individuals with mental disorders or COPD. The conditional recommendation suggests combining varenicline with a nicotine patch instead of using varenicline alone. Aligned with the Korean Society of Medicine’s clinical guideline development process, this is South Korea’s first domestic smoking cessation treatment guideline that follows standardized guidelines. Primarily focusing on pharmacotherapy, it can serve as a foundation for comprehensive future smoking cessation clinical guidelines, encompassing broader treatment topics beyond medications.
INTRODUCTION
Smoking is one of the greatest public health threats worldwide, causing over 8 million deaths annually, including approximately 1.2 million deaths from secondhand smoke exposure. Cigarettes have detrimental effects on nearly all organs of the body, leading to malignant tumors, cardiovascular diseases, and respiratory disorders. The Global Burden of Disease Study, published in 2020 using data from 2019, estimated a total of 8.71 million deaths worldwide due to tobacco use, with the highest mortality rate attributed to smoking among males [1]. Regarding public health significance, the World Health Organization (WHO) adopted the Framework Convention on Tobacco Control in 2003, which officially went into force in 2005. Article 14 of this convention recommends comprehensive guidelines based on scientific evidence for development and dissemination [2]. Consequently, countries like the United States, the United Kingdom, and Australia have developed and published national smoking cessation guidelines following this agreement [3].
In South Korea, awareness of the risks of smoking has been improved and reinforced through enhanced anti-smoking policies, resulting in a nearly 50% reduction in smoking rates from 1998 to 2020 [4]. However, the decline in male smoking rates has slowed since 2008. The female smoking rate has remained relatively stable over the past 20 years, indicating the need for further management. While clinical guidelines are developed by expert organizations such as the Korean Academy of Family Medicine, the Korean Society of Tuberculosis and Respiratory Diseases, and the Korean Medical Association, no national recommendations strictly follow developing clinical treatment guidelines [5]. To address this, a multidisciplinary committee for smoking cessation clinical guidelines was established to provide recommendations based on domestic and international research findings and guidelines tailored to the domestic environment. This study aimed to assist frontline clinicians involved in smoking cessation support programs in dealing with the issues that may arise when initiating pharmacotherapy for smoking cessation.
GUIDELINE SCOPE AND TARGET AUDIENCE
In this recommendation, smoking cessation pharmacotherapy is targeted at adults aged 19 years and above who are approved for such treatment in South Korea. Pregnant women and adolescents for whom smoking cessation pharmacotherapy was not indicated in the country were excluded. This guideline aims to reduce unnecessary variations in practice and provide an evidence-based approach in particular circumstances. This guideline is prepared for all physicians working in National Health Insurance Service-funded clinical settings where pharmacological therapy is provided. The target audience for the recommendations in our guidelines includes patients, physicians, nurses, and policymakers who inform patient decision-making, clinical practice, and health policy decisions.
DISCLAIMER
Considering the domestic situation, we considered the balance between desirable and undesirable effects, certainty of evidence, patient values and preferences, required resources, equity, acceptability, and feasibility for all recommendations. These guidelines do not replace professional judgment in particular cases wherein the clinician or health professional may decide that individual guideline recommendations are inappropriate for the circumstances presented by an individual patient.
METHODS
The development methodology adopted for this recommendation is the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-ADOLOPMENT approach [6]. It involves searching, selecting, and reviewing major domestic and international guidelines and conducting a systematic literature review to assess the quality and currency of the evidence. Based on the acceptance, modification, or newly identified literature for each recommendation, decisions were made on adopting the existing guidelines or developing new ones. A schematic flow of the methodology is shown in the Supplementary figure (Supplement 1). Panel composition, conflict of interest management, and external review were performed per the Korean Academy of Medical Sciences policies and procedures [7].
1. Guideline Development Group Composition
Potential members were identified based on their expertise and training in tobacco dependence counseling and/or treatment. The committee comprises family medicine, psychiatry, pulmonology, and cardiology experts to promote multidisciplinary collaboration. The committee also included experts on the methodology.
2. Conflict of Interests Management
All members disclosed potential conflicts of interest to the committee and were determined to have no substantial conflicts of interest. Examples of potential conflicts of interest include financial support from or connections with companies, political pressure from interest groups, and academic issues.
3. Questions and Outcomes of Interest
Based on searchable smoking cessation treatment guidelines and systematic literature reviews by the Cochrane Group, the committee selected 14 topics regarding pharmacological treatments for smoking cessation to derive key questions. The committee has considered various issues that have consistently been raised in smoking cessation support programs, including the prioritization of smoking cessation medication selection, evidence for combination therapy, methods of smoking cessation treatment (prolonged treatment, pre-cessation medication, flexible quit dates), smoking cessation for novel tobacco products, the effectiveness of smoking cessation in special populations (chronic obstructive pulmonary disease [COPD], mental disorders, cardiovascular diseases, women, national lung cancer screening program, hospitalized patients), and other related issues. After selecting the issues, the development committee conducted meetings to choose seven questions relevant to the domestic healthcare reality. Considering the expertise of the development committee members, responsible working-group members were assigned each question to address their specific content. Each question was organized in the “Population, Intervention, Comparator, Outcome” (PICO) format. For the outcome variables, the committee selected (1) long-term abstinence rate after 6 months of smoking cessation (self-reported/biochemically validated, 7-day point prevalence abstinence/continuous abstinence) and (2) serious adverse events.
4. Literature Search
The search was conducted by librarians using the following search engines: Ovid-MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, and KoreaMed. The working group members were responsible for each topic, and the search experts selected the appropriate search terms. A literature search was performed for each topic, covering publications published until November 30, 2022. A search for international guidelines on smoking cessation was conducted to identify recommendations. The details of the search strategies are described in a separate file (Supplement 2).
5. Literature Screening and Evidence Synthesis
The common selection criteria for the literature search were as follows: (1) documents written in English or Korean; (2) studies targeting adults; and (3) guidelines, systematic literature reviews, meta-analyses, and randomized controlled trials (RCTs). The exclusion criteria were as follows: (1) documents written in languages other than English or Korean; (2) studies targeting children or adolescents; (3) cases where the full text of the document was unavailable; and (4) non-representative single-author clinical guidelines and similar sources.
For studies that matched the key questions and had clear guidelines with recommendation grades and an evidence-to-decision framework, at least two committee members used the AGREE II tool (The Appraisal of Guidelines for Research and Evaluation, https://www.agreetrust.org/) [8] to evaluate the quality of the development methods for each guideline. For literature reviews, the AMSTAR 2 tool (A Measurement Tool to Assess Systematic Reviews) [9] was used to evaluate quality. If relevant guidelines or systematic literature reviews were available for each key topic, the committee either adopted or revised the content to ensure currency and relevance. When no guidelines or systematic literature reviews were available, the committee selected relevant RCTs from the literature. Meta-analyses were conducted based on selected literature. RCTs were evaluated using Cochrane’s risk of bias (RoB) tool (Cochrane, London, UK) [10]. Two assigned committee members independently conducted the initial assessment; in cases where the results were inconsistent, the committee chairperson and members discussed and reached a final evaluation.
Based on the literature search results, the committee adopted the most appropriate recent guidelines from the American Thoracic Society (ATS). Initiating pharmacologic treatment in tobacco-dependent adults (2020) [11], which aligns with the PICO of the key question. The AGREE II tool was applied to evaluate these clinical guidelines, specifically focusing on the eight items related to the rigor of development in evaluation domain 3. Two evaluators independently assessed each item. Each evaluator scored each AGREE II evaluation tool item on a 7-point scale. Subsequently, scores were calculated for each domain and converted to percentages. The evaluators then engaged in discussions to reach a consensus on decision-making based on the results. A systematic literature review was conducted using the criteria provided by AMSTAR 2. After converting domain three scores into percentages and obtaining a score of 70.8%, the committee reached a consensus regarding the suitability of the ATS guidelines. Given that the PICO of the key question aligns with the ATS guidelines, the ATS guidelines were adopted.
6. Formulating Recommendations
For each key topic, the committee utilized The GRADE methodology to determine the level of evidence [6]. The level of evidence refers to the degree of confidence in the effectiveness of a specific intervention based on available evidence. It is subdivided into four grades (high, moderate, low, and very low) and considers factors such as the RoB, consistency, directness, precision, and publication bias (Table 1). The strength of a recommendation refers to the degree of confidence that the benefits of an intervention outweigh its harm (or vice versa) when implemented in target patients. It considers the level of evidence, magnitude of benefits and harms, resource utilization, and patient preferences and values. It is classified into four categories: strong for, weak for, weak against, and strong against (Table 2). The “evidence-to-decision tables” are attached as a supplementary file (Supplement 3).
7. Independent Review
The Korean Society for Research on Nicotine and Tobacco (KSRNT, http://ksrnt.org/main/main.php) document editors and anonymous peer reviewers reviewed the recommendations. They were approved for publication by the KSRNT, following the organization’s policy.
8. Funding
The National Health Insurance Service funded these guidelines (https://www.nhis.or.kr/nhis/index.do). The National Health Insurance Service did not influence the contents of the guidelines.
QUESTIONS AND RECOMMENDATIONS
The primary reasons smokers find it difficult to quit smoking are nicotine withdrawal symptoms and cravings. Major smoking cessation guidelines recommend pharmacotherapy to increase the success rate of smoking cessation. Therefore, pharmacotherapy is recommended for smokers without specific contraindications. This recommendation provides information on the priority order for selecting smoking cessation medications, treatment methods (combination therapy, treatment duration, and pre-cessation medication), and medication therapy for special populations (such as those with COPD or mental disorders).
1. General Population
1) Which medication, varenicline or nicotine patch, should be used as the primary treatment in smoking cessation therapy?
Questions 1 and 2 aimed to provide evidence for selecting the optimal monotherapy among nicotine patch, varenicline, and sustained-release bupropion for smoking cessation. In main question 1, the smoking abstinence rates of 6 months or longer and serious adverse events were compared between nicotine patch and varenicline in smokers aged 19 years and above.
Recommendation: For smoking cessation treatment, we recommend varenicline over a nicotine patch. (Strong recommendation, moderate certainty)
The recommendation from the ATS titled “Initiating pharmacologic treatment in tobacco-dependent adults, 2020” was evaluated based on the AGREE II criteria and adopted [11]. A meta-analysis of 11 RCTs found that varenicline had a higher smoking cessation success rate at 6-month follow-up, as self-reported or confirmed by exhaled carbon monoxide, compared to nicotine patch (risk ratio [RR], 1.20; 95% confidence interval [CI], 1.09 to 1.32) [12-22]. Additionally, varenicline did not increase the risk of serious adverse events compared to Nicotine Patch (RR, 0.72; 95% CI, 0.52 to 1.00) (Table 3). Although the main question is compared to monotherapy, combination therapy with sustained-release formulations such as patches and immediate-release formulations such as gums or lozenges, in addition to “nicotine replacement therapy (NRT) monotherapy,” can increase smoking cessation success rates by 25% compared to monotherapy alone [23]. Therefore, when initiating NRT treatment, combination therapy can be chosen, and the nicotine dosage of the nicotine patch or gum can be adjusted based on the level of nicotine dependence.
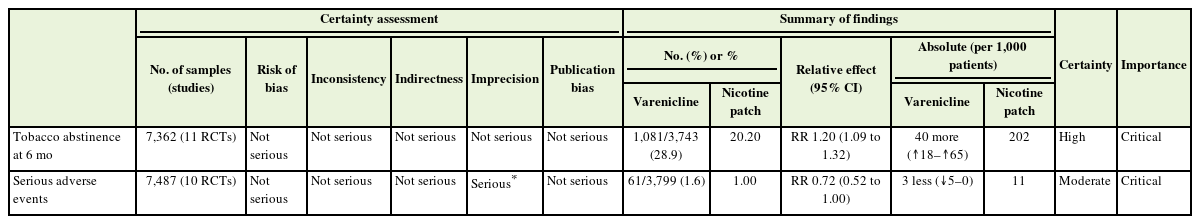
Evidentiary basis for strong recommendation favoring varenicline over nicotine patch, with moderate-certainty evidence
2) Which medication, varenicline or Bupropion, should be used as the primary treatment in smoking cessation therapy?
Bupropion works by inhibiting the reuptake of dopamine and norepinephrine at nerve terminals, which helps alleviate withdrawal symptoms and reduce cravings [24]. It is an effective medication for smoking cessation, regardless of the presence of depression. In main question 2, the smoking cessation success rates of 6 months or longer and serious adverse events were compared between bupropion and varenicline in smokers aged 19 years and above.
Recommendation: For smoking cessation treatment, we recommend varenicline over a bupropion. (Strong recommendation, moderate certainty)
The ATS recommendation (2020) was evaluated based on the AGREE II criteria and adopted [11]. Varenicline was found to have a higher smoking cessation success rate at 6 months compared to bupropion sustained-release (RR, 1.30; 95% CI, 1.19 to 1.42), with no significant difference in the occurrence of serious adverse effects (RR, 0.81; 95% CI, 0.57 to 1.16) (Table 4) [13,25-30]. In a subsequent network meta-analysis reported in 2022, comparing varenicline, NRT, and bupropion, varenicline was reported as the most effective monotherapy [31]. However, sufficient information regarding efficacy and side effects should be provided to the patient when initiating smoking cessation treatment, taking into consideration the patient’s preferences.
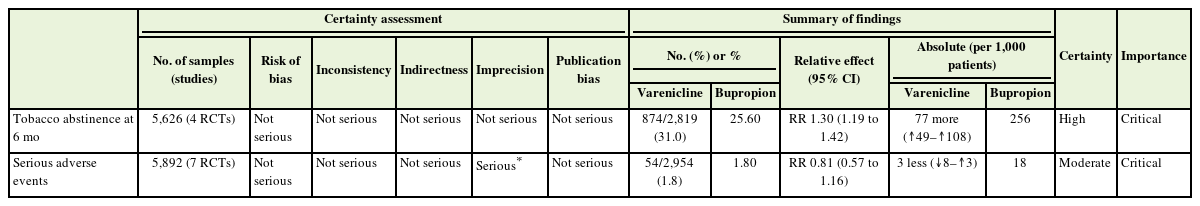
Evidentiary basis for strong recommendation favoring varenicline over bupropion, with moderate-certainty evidence
3) In smoking cessation therapy, should the treatment be initiated with the combination therapy of varenicline and NRT or the monotherapy of varenicline alone?
In the guidelines provided by the ATS, the combination of varenicline and nicotine adjunctive therapy was reviewed and recommended as more effective than varenicline monotherapy for smoking cessation. After the inclusion of studies by Ramon et al. [32] and Koegelenberg et al. [33], followed by the publication of a study by Baker et al. [34] in 2021, this guideline included a meta-analysis of three RCTs. In smokers aged 19 years and older, smoking cessation success rates of 6 months or longer and significant adverse events were compared between the combination therapy of varenicline and nicotine adjunctive therapy and varenicline monotherapy.
Recommendation: For smoking cessation treatment, we conditionally suggest varenicline plus a nicotine patch over varenicline alone. (Weak recommendation, moderate certainty)
The analysis results showed that the combination therapy of varenicline and NRT improved the smoking cessation success rate by 27% compared to varenicline monotherapy (RR, 1.27; 95% CI, 1.11 to 1.46). There was no significant difference in the occurrence of serious adverse events between the two groups (RR, 0.83; 95% CI, 0.24 to 2.89) (Figure 1, Table 5). However, the combination therapy of varenicline and NRT may have lower acceptability from the perspective of healthcare providers prescribing smoking cessation medications, patients, and stakeholders involved in smoking cessation therapy. In addition, there is limited evidence regarding the cost-effectiveness of combination therapies. Therefore, it may be more acceptable to gradually introduce combination therapy rather than starting from the beginning. Therefore, combination therapy is recommended, particularly if monotherapy alone does not achieve sufficient smoking cessation or symptom relief. Further studies on cost-effectiveness and relapse prevention effects are needed, and different effects depending on the type of nicotine replacement agent should be evaluated.
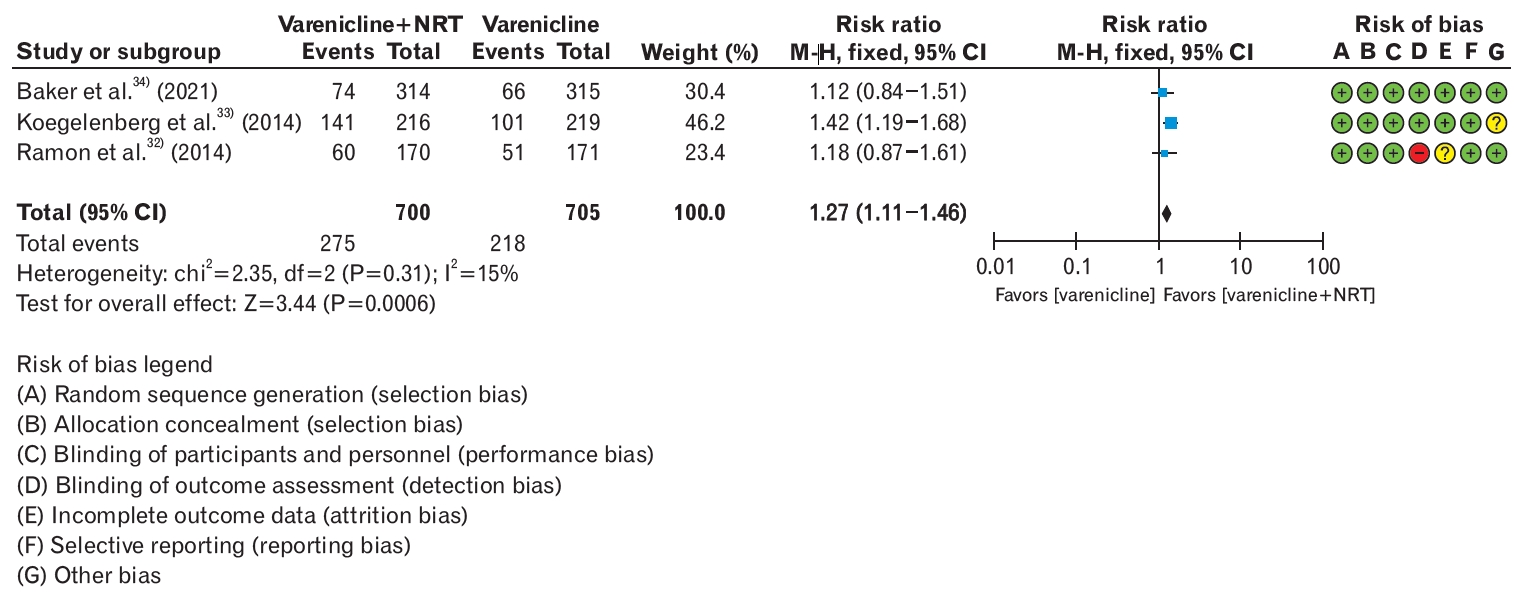
Smoking cessation rate in varenicline plus nicotine patch versus varenicline only. Events were defined as smoking cessation success of 6 months or longer. Risk ratios were calculated using the M-H method to combine summary statistics, and data were pooled using a fixed-effects model. NRT, nicotine replacement therapy; M-H, Mantel-Haenszel test; CI, confidence interval; df, degrees of freedom; I2, percentage of variation across studies due to heterogeneity.

Evidentiary basis for conditional recommendation favoring varenicline plus patch over patch alone, with low-certainty evidence
4) In smoking cessation therapy, is extended treatment (12 weeks or longer) more effective than standard treatment (8–12 weeks)?
The smoking cessation support program consisted of a first phase of 12 weeks, with the option of extending the program up to three additional times if necessary. Research studies have reported that extending pharmacotherapy is effective [35]. In this key question, the smoking cessation success rates of 1 year or longer and serious adverse events were compared between extended treatment (>12 weeks) and standard treatment (8–12 weeks) in smokers aged 19 years and above.
Recommendation: For smoking cessation treatment, we recommend using extended-duration (>12 weeks) over standard-duration (8–12 weeks) therapy. (Strong recommendation, moderate certainty)
A systematic literature review study published in 2022 was evaluated based on the AMSTAR 2.0 criteria and adopted [36]. The meta-analysis, which combined nine RCTs, showed that extended treatment improved the smoking cessation success rate by 18% compared to standard treatment (RR, 1.18; 95% CI, 1.05 to 1.33) [34,37-44]. There was no significant difference in the occurrence of serious adverse events between the two groups (RR, 1.37; 95% CI, 0.79 to 2.36) (Figure 2, Table 6).

Smoking cessation rate in extended over standard-duration of treatment[36]. Events were defined as the 7-day point of prevalent abstinence at 12 months. Risk ratios were calculated using the M-H method to combine summary statistics, and data were pooled using a fixed-effects model. M-H, Mantel-Haenszel test; CI, confidence interval; df, degrees of freedom; I2, percentage of variation across studies due to heterogeneity.

Evidentiary basis for strong recommendation favoring extended over standard-duration treatment of regimens, with moderate-certainty evidence
In general, pharmacotherapy for smoking cessation is recommended for a minimum of 3 months. The committee members assessed that extended treatment was acceptable to policy stakeholders, such as the National Health Insurance Service and the Ministry of Health and Welfare. In South Korea, smoking cessation support programs already provide support for up to three sessions per year, and gradual cessation methods that offer treatment for up to 24 weeks have been approved for varenicline. Cost-effectiveness analyses of extended treatment were conducted for varenicline based on two systematic literature review studies, which reported that extended treatment is costeffective [45,46]. Future cost-effectiveness analyses based on domestic data are also necessary for the stable operation of smoking cessation support programs. Varenicline and bupropion can be extended for up to 1 year if individuals still perceive a delayed risk of relapse from previous attempts. In cases where bupropion is used for mood regulation, the extent of treatment should consider the management of depressive symptoms [37]. NRT can be extended if necessary.
5) Is smoking cessation pharmacotherapy effective for smokers who are not ready to quit smoking?
The transtheoretical stage-of-change model is used in most treatment strategies to assess readiness to quit smoking [47]. However, unlike this model, many smokers exhibit dynamic smoking cessation behaviors, such as quitting without a specific plan and resisting smoking urges [48]. Therefore, providing pre-cessation pharmacotherapy to smokers before they quit can help maintain abstinence [49]. In key question 5, we compared the 6-month or longer abstinence rates and serious adverse events of pre-cessation treatment with varenicline and placebo in smokers aged 19 years and above who were not yet ready to quit smoking.
Recommendation: In tobacco dependent adults who are not ready to discontinue tobacco use, we recommend that clinicians begin treatment with varenicline rather than waiting until patients are ready to stop tobacco use. (Strong recommendation, moderate certainty)
The ATS recommendation (2020) was evaluated based on the AGREE II criteria and adopted [11]. A meta-analysis of three RCTs showed that pre-treatment with varenicline significantly improved the smoking cessation success rate by 100% compared to placebo (RR, 2.00; 95% CI, 1.70 to 2.35) [50-52]. There was no significant difference in serious adverse events between the two groups (RR, 1.77; 95% CI, 0.98 to 3.13) (Table 7). Therefore, pre-treatment with smoking cessation medications, specifically varenicline, is recommended for smokers with nicotine dependence. Pre-treatment with varenicline aims to address the craving for smoking rather than treating the smoking behavior itself. This can help increase motivation for smoking cessation among smokers who want to quit but are unable to do so immediately. However, the appropriate pre-treatment duration with medication is not yet known, and further research is needed. The panel acknowledged the potential threat to patient autonomy when a proactive approach was misapplied. Patient autonomy can be preserved when clinicians engage with their patients in discussions, encourage pharmacotherapy with continued smoking, and respect their decision to decline treatment.
2. Special Population
1) Is pharmacotherapy effective for smoking cessation in patients with mental disorders, including substance use disorder, depression, anxiety, schizophrenia, and/or bipolar disorder?
Compared with the general population, individuals with mental disorders have a significantly higher smoking rate. However, evidence regarding the effectiveness of smoking cessation pharmacotherapy in patients with mental illness is limited. Additionally, information on the safety of varenicline and other medications is lacking, with varenicline previously carrying a black-box warning for neuropsychiatric adverse events (issued by the US Food and Drug Administration [FDA] and European Medicines Agency). To compare the 6-month or longer abstinence rates and significant adverse events of varenicline with NRT in individuals aged 19 years and older with mental illnesses who smoke, a systematic literature review was conducted.
Recommendation: For smoking cessation treatment in adults with comorbid mental disorders, we recommend varenicline over a nicotine patch. (Strong recommendation, high certainty)
Based on a meta-analysis of six RCTs, varenicline significantly improved smoking cessation rates by 38% in patients with mental illnesses compared to NRT (RR, 1.38; 95% CI, 1.27 to 1.50), with no significant difference in serious adverse events (RR, 1.07; 95% CI, 1.00 to 1.15) (Figure 3, Table 8) [13,18,53-56]. Both in populations with mental illnesses and those without, varenicline was found to be the most effective and safe treatment for smoking cessation. There was no evidence to support a higher incidence of neuropsychiatric adverse events with varenicline or bupropion than with NRT alone. Since the removal of the black-box warning for varenicline by the US FDA in December 2016, concerns regarding adverse psychiatric events have significantly diminished. However, there is still a passive approach to smoking cessation treatment in individuals with mental illnesses such as alcohol use disorder, depression, bipolar disorder, and schizophrenia in the domestic setting. Given the higher smoking rates among populations with mental illness, there is a need to actively provide information to healthcare professionals and patients to encourage the use of smoking cessation pharmacotherapy.
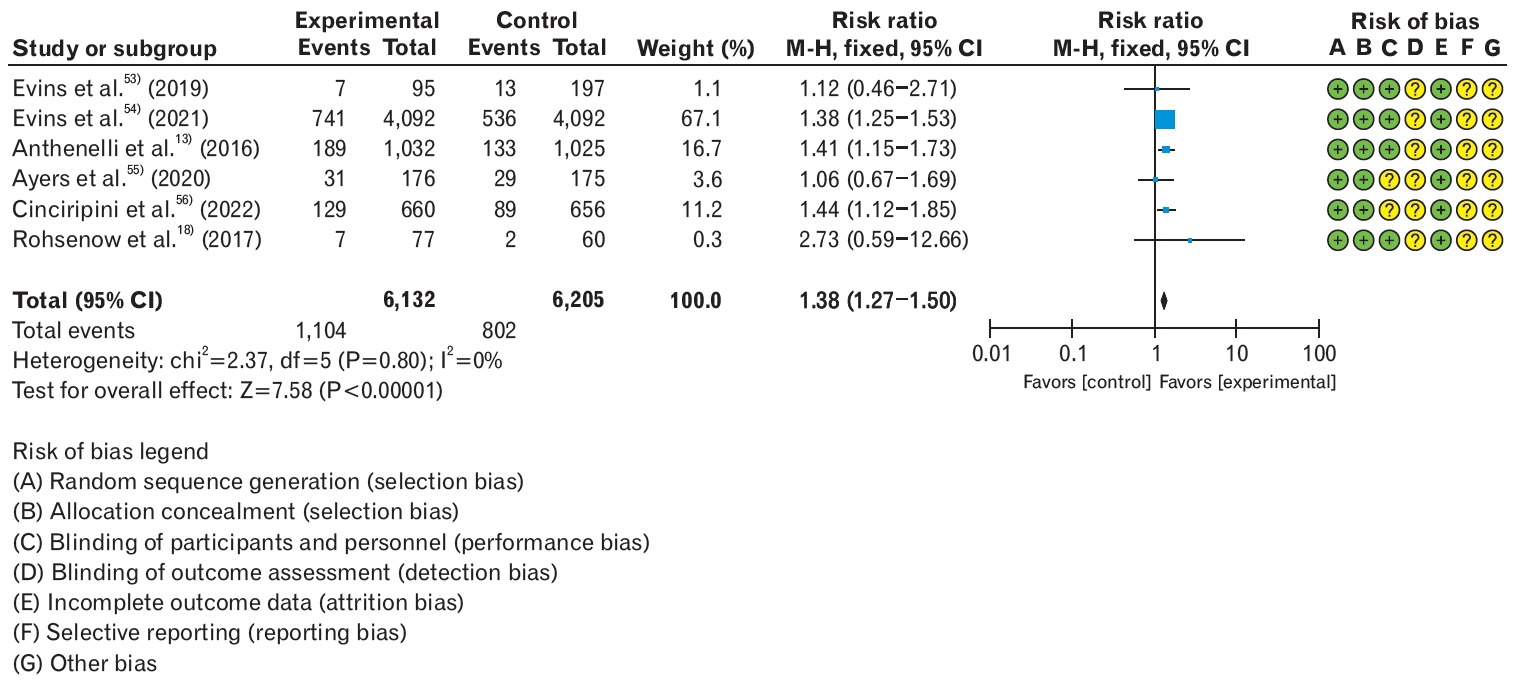
Smoking cessation rate in varenicline over nicotine patch in patients with comorbid mental disorders. Events were defined as smoking cessation success of 6 months or longer. Risk ratios were calculated using the M-H method to combine summary statistics, and data were pooled using a fixed-effects model. M-H, Mantel-Haenszel test; CI, confidence interval; df, degrees of freedom; I2, percentage of variation across studies due to heterogeneity.

Evidentiary basis for strong recommendation favoring varenicline over nicotine patch in patients with mental disorders, with high-certainty evidence
2) Is pharmacotherapy effective for smoking cessation in patients with chronic obstructive pulmonary disease?
Smoking cessation is the most effective treatment for delaying COPD progression. It reduces exacerbation and hospitalization rates and facilitates disease management, ultimately improving patients’ quality of life [57]. According to the literature, 38%–77% of patients with COPD continue to smoke even after being diagnosed with the disease, highlighting the importance of effective smoking cessation treatment in this population [58]. Therefore, a systematic literature review was conducted to compare the long-term smoking cessation rates and serious adverse events of varenicline, bupropion, or NRT in patients with COPD who smoke.
Recommendation: For smoking cessation treatment in adults with COPD, we recommend pharmacotherapy, including varenicline, bupropion, or NRT. (Strong recommendation, moderate certainty)
Based on the analysis of five RCTs, it was found that pharmacological treatment with varenicline, bupropion, or NRT was more effective in achieving smoking cessation among patients with COPD compared to the placebo group (RR, 2.24; 95% CI, 1.67 to 3.01) [59-63]. Furthermore, the risk of serious adverse events did not significantly increase with medication therapy for smoking cessation in these patients (RR, 0.67; 95% CI, 0.4 to 1.02) (Figure 4, Table 9). Although acute exacerbations are commonly associated with COPD, further large-scale studies are needed to determine the effectiveness of medication therapy during acute exacerbations. Smoking cessation treatment in patients with COPD is essential for preventing disease exacerbation and lung cancer and does not pose significant risks of serious adverse effects. Therefore, it is important to disseminate this information to clinicians and patients to emphasize the integration of smoking cessation treatments with disease management.
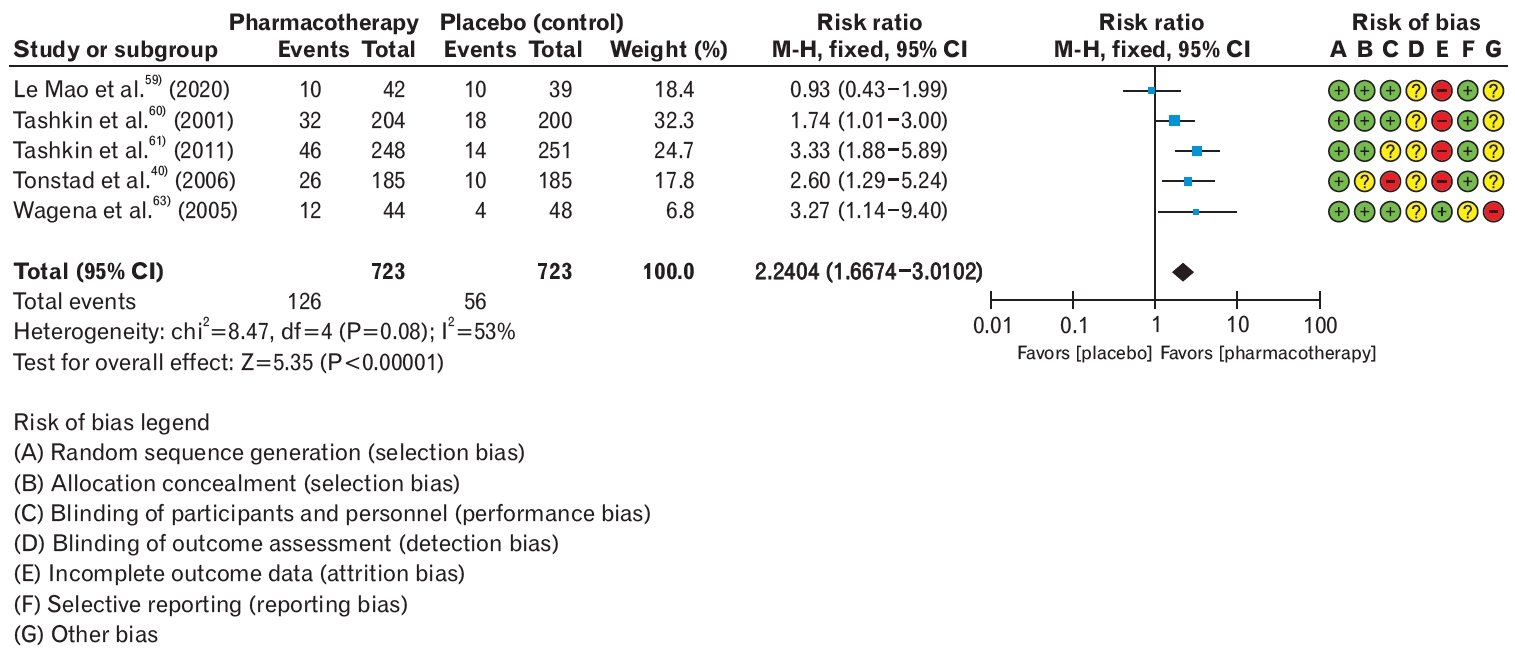
Smoking cessation rate in pharmacotherapy (varenicline, bupropion, or nicotine replacement therapy) over placebo in patients with chronic obstructive lung disease. Events were defined as smoking cessation success of 6 months or longer. Risk ratios were calculated using the M-H method to combine summary statistics, and data were pooled using a fixed-effects model. M-H, Mantel-Haenszel test; CI, confidence interval; df, degrees of freedom; I2, percentage of variation across studies due to heterogeneity.
DISCUSSION
Tobacco smoking is recognized as the leading preventable cause of death. The WHO recommends developing and disseminating comprehensive, evidence-based smoking cessation guidelines in each country (Article 14 of the WHO Framework Convention on Tobacco Control). While 80% of the major countries have developed and disseminated national smoking cessation guidelines, in Korea, development has been limited to individual societies or specialized organizations that have created their recommendations. Furthermore, smoking cessation clinical practice guidelines were not developed based on the clinical practice guideline development process of the Korean Association of Medical Sciences. Indeed, the expansion of electronic cigarettes, heated tobacco, and the coronavirus disease 2019 pandemic have led to a decrease in the number of individuals visiting smoking cessation clinics. Evidence-based clinical practice guidelines are essential to initiate smoking cessation treatments. These guidelines were developed with the support of the National Health Insurance Service to provide consistent solutions for issues that frontline healthcare providers may encounter when initiating pharmacological treatment for smoking cessation under the Smoking Cessation Treatment Support Program that has been implemented since 2015.
This guideline for smoking cessation treatment adheres to the process of developing clinical guidelines by the Korean Medical Association. It is specifically developed for frontline clinicians in outpatient clinics who provide smoking cessation services. The development committee, composed of multidisciplinary experts, selected the scope and target population of the guidelines, formulated key questions, conducted a literature search and selection, synthesized and evaluated the evidence, determined the recommendation grades, and finalized the seven recommendations by incorporating external reviews. These guidelines address several limiting misconceptions, including the value of combination pharmacotherapy, the approach to patients who are reluctant to stop smoking, and the safety and efficacy of treating patients with vulnerable behavioral health.
This guideline focuses on pharmacological treatments for smoking cessation. It is anticipated that future national clinical guidelines on smoking cessation will be developed, encompassing more comprehensive topics to be provided to healthcare professionals involved in smoking cessation counseling and patient care.
SUMMARY OF RECOMMENDATION
1. We recommend varenicline over nicotine patches for smoking cessation. (Strong recommendation; moderate certainty)
2. For smoking cessation, we recommend varenicline over bupropion. (Strong recommendation; moderate certainty)
3. For smoking cessation, we recommend varenicline plus a nicotine patch over varenicline alone. (Weak recommendation; moderate certainty)
4. For smoking cessation treatment, we recommend using extended therapy (>12 weeks) over standard therapy (8–12 weeks). (Strong recommendation; moderate certainty)
5. In tobacco-dependent adults who are not ready to discontinue tobacco use, we recommend that clinicians begin treatment with varenicline rather than waiting until patients are ready to stop tobacco use. (Strong recommendation; moderate certainty)
6. We recommend varenicline over a nicotine patch for smoking cessation in adults with comorbid psychiatric conditions. (Strong recommendation; high certainty)
7. For smoking cessation treatment in adults with COPD, we recommend pharmacotherapy with varenicline, bupropion, or NRT. (Strong recommendation; moderate certainty)
Notes
CONFLICT OF INTEREST
Soo Young Kim, one of the authors of this article, serves as an editor for the Korean Journal of Family Medicine. To ensure transparency and mitigate any potential conflicts of interest, the peer review and editorial process for this article were managed entirely by independent editors of the journal. Soo Young Kim was not involved in the editorial decision- making process, including the peer review, acceptance, or rejection of this manuscript. Except for that, no potential conflict of interest relevant to this article was reported.
Acknowledgements
These guidelines were developed with research funding from the National Health Insurance Service. We would like to express our gratitude to the Ministry of Health and Welfare’s Health Promotion Division for their assistance in establishing and conducting this research, as well as to the Smoking Cessation Support Team of the National Health Insurance Service and the staff at the National Evidence-Based Healthcare Collaborating Agency for their invaluable contributions.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.23.0142. Supplement 1. Development process for the CPG for smoking cessation using GRADE-ADOLOPMENT. Supplement 2. Search strategy. Supplement 3. Evidence to decision.
Development process for the clinical practice guidelines for smoking cessation using GRADE (Grading of Recommendations Assessment, Development, and Evaluation)-ADOLOPMENT. ETD, evidence to decision.
Search strategy
Evidence to decision




