Association between Obstructive Sleep Apnea and Glaucoma
Article information
Abstract
Background
Obstructive sleep apnea (OSA) and glaucoma are major global health challenges. However, the probable association between them is yet to be fully elucidated. This study aimed to investigate the association between OSA and glaucoma.
Methods
Data for this cross-sectional study were obtained from the eighth Korea National Health and Nutrition Examination Survey (2019–2021). From among 9,495 individuals who completed the STOP-Bang questionnaire on OSA (for those aged ≥40 years) and provided their glaucoma prevalence/intraocular pressure (IOP) data, 8,741 were selected for glaucoma prevalence analysis. A total of 754 individuals aged 80 years or older or those with missing confounding variable data were excluded. A separate subgroup of 8,627 individuals was selected for IOP analysis after excluding 114 individuals who use glaucoma eye drops. The study employed linear and logistic regression analyses with Stata/MP ver. 17.0 (Stata Corp., USA) to understand the relationship between the risk of OSA assessed using the STOP-Bang score and key glaucoma indicators, adjusted for confounders. Statistical significance was set at a P-value <0.05.
Results
The average±standard deviation [SD] age of the glaucoma prevalence study group was 56.59±10.48, and 42.98% were male. Notably, every unit increase in the STOP-Bang score was associated with a greater risk of glaucoma (odds ratio, 1.097; P=0.044). In the IOP subgroup, the average±SD age was 56.49±10.45 years, with 42.88% being males. The linear regression showed a statistically significant relationship between the STOP-Bang score and IOP after adjusting for confounding variables (β=0.171, P<0.001).
Conclusion
Our findings revealed a significant positive association between OSA risk, as measured using the STOP-Bang score, and both the likelihood of glaucoma and high IOP.
INTRODUCTION
Glaucoma is a leading cause of irreversible blindness, affecting more than 70 million people worldwide, and is characterized by progressive optic nerve damage and changes in the visual field [1]. Although elevated intraocular pressure (IOP) is a well-established risk factor for glaucoma, its pathogenesis is not fully understood [2]. In normotensive glaucoma (NTG), a high IOP does not necessarily lead directly to glaucoma [3]. Thus, other factors such as age, family history of glaucoma, ethnicity, thin corneas, and certain systemic conditions including diabetes and cardiovascular disease also contribute to its development [2].
Obstructive sleep apnea (OSA) is a common sleep disorder that affects approximately 20% of adults. It is characterized by intermittent hypoxemia and arousal due to recurrent episodes of complete upper airway collapse during sleep [4]. OSA is associated with various adverse health outcomes including cardiovascular diseases, metabolic disorders, and neurocognitive impairment [5]. Given the shared risk factors of OSA and glaucoma, including old age, obesity, and systemic comorbidities, the potential association between the two conditions has gained attention in recent years [6]. Studies have suggested that patients with OSA are at a higher risk of glaucoma than those without OSA [7], emphasizing the need for appropriate screening. Although several conditions, including inflammation, oxidative stress, hypoxia, and sympathetic tone, show a possible association between OSA and glaucoma [6], the precise nature of this association and the underlying pathophysiological mechanisms remain unclear and inconclusive.
The STOP-Bang score is a simple survey tool used to assess the risk of OSA, with higher scores indicating greater risk of OSA [8]. Recently, the applicability of the STOP-Bang score has expanded, not only to assess OSA risk but also to gauge the severity of OSA; however, further research is required. Although some previous studies have explored the association between glaucoma and OSA [9,10], only a few have examined the risk of OSA in glaucoma patients using the STOP-Bang score [11,12]. In particular, only a few studies have investigated the association between OSA risk and severity using the STOP-Bang score and the risk of glaucoma or increased IOP using Korean national data.
This study used data from the eighth Korea National Health and Nutrition Examination Survey (KNHANES) to investigate the association between OSA risk, as measured by the STOP-Bang score, a possible detector of individuals at high risk for glaucoma, and both the prevalence of glaucoma and IOP.
METHODS
1. Data Sources
This study utilized data from the eighth KNHANES, a nationwide population-based cross-sectional health examination and survey conducted by the Korea Disease Control and Prevention Agency (formerly, Korea Center for Disease Control and Prevention). The KNHANES collects comprehensive health information from a large sample of the Korean population using a complex, multistage, stratified, and clustered sampling design. The surveys included interviews, health examinations, and nutritional surveys. All survey protocols were approved by the Institutional Review Board (IRB) of the Korea Disease Control and Prevention Agency (IRB approval numbers: 2018-01-03-C-A, 2018-01-03-2C-A, 2018-01-03-5C-A).
2. Study Population
This study included participants who underwent a health examination component of the eighth KNHNES between 2019 and 2021. The study population consisted of individuals who completed both the sleep hygiene section, including the STOP-Bang questionnaire (age ≥40 years), and the section related to ophthalmology, which included information on glaucoma and IOP. Participants with missing data on comorbidities or potential covariates were excluded from the analysis.
3. Variable Definition
Body mass index (BMI) was defined as body weight in kilograms divided by the square of the height in centimeters (kg/cm2). Current smoking status was defined as ‘yes’ if someone was a current smoker or had smoked more than five packs (equivalent to 100 cigarettes) in their lifetime, and ‘no’ otherwise.
1) Variables assessed
The likelihood of OSA was assessed in participants aged ≥40 years using the STOP-Bang questionnaire, a validated screening tool for OSA [13]. The STOP-Bang score ranges from 0 to 8, with higher scores indicating a greater risk or severity of OSA [8]. The questionnaire comprises questions related to snoring (S, “Do you snore loudly, louder than talking or loud enough to be heard through closed doors?”), tiredness (T, “Do you often feel tired, fatigued, or sleepy during the daytime?”), observation (O, “Has anyone observed you stop breathing during sleep?”), hypertension (P, “Do you have [or are you being treated for] high blood pressure?”), BMI >35 kg/m2 (B), age >50 years (A), neck circumference >40 cm (N), and male sex (G). Each question was scored as 1 if the participant answered “yes” and 0 if the participant answered “no.” The scores were then summed to obtain a final score ranging from 0 to 8.
2) Components of the ophthalmic survey and examinations
The survey was conducted among participants aged ≥40 years in 2019 and 2020 and those aged 10–59 years in 2021. These participants completed ophthalmic questionnaires that included a history of recent ocular examinations, ophthalmic surgery, presence of conditions such as cataracts, age-related macular degeneration, glaucoma, diabetic retinopathy, along with inquiries about contact lens wear, and near-work duration. They also underwent ophthalmological examinations that evaluated visual acuity, autorefraction, fundus photography, axial length, optical coherence tomography, IOP, and the visual field [14].
3) Intraocular pressure
The IOP was measured with the participants in a seated position using rebound tonometry (Icare PRO; Icare Finland Oy, Helsinki, Finland), and the average of six readings was automatically generated. The average IOP of both eyes was used as the representative value, and color indicators such as green, yellow, and red were used to signify the reliability of the measurements and potential deviations or errors.
4) Glaucoma status
The participants were diagnosed with various types of glaucoma, including open-angle and angle-closure glaucoma, based on detailed criteria involving visual field tests, optical coherence tomography scans, IOP measurements, and structural changes in the eye. Diagnoses of glaucoma-related diseases and retinal diseases, such as diabetic retinopathy and age-related macular degeneration, were made by experienced specialists and verified through a double-reading process, and any disagreements were resolved by an independent senior reading committee. Further details related to the ophthalmic survey and measurements are described in detail in a previous study [14].
5) Hypertension-related variables
If an individual is taking antihypertensive drugs and has a systolic blood pressure (SBP) ≥140 mm Hg, or a diastolic blood pressure (DBP) ≥90 mm Hg, they were classified as having hypertension. “Prehypertension” was defined by a SBP between 120 mm Hg and 139 mm Hg or a DBP between 80 mm Hg and 89 mm Hg, provided that the person was not already classified as having “hypertension.” If someone did not meet the criteria for either “hypertension” or “prehypertension” and had a SBP <120 mm Hg and a DBP <80 mm Hg, they were classified as “normal.” Blood pressure measurements were performed 3 times, using the average of the latter two as representative values.
6) Diabetes-related variables
Individuals who had fasted for more than 8 hours (excluding pregnant women), those diagnosed with diabetes mellitus, taking oral hypoglycemic agents or insulin injections, or having a fasting blood glucose level ≥126 mg/dL or a hemoglobin A1c (HbA1c) level ≥6.5% were classified as having diabetes. The “prediabetes” category encompassed those with a fasting blood glucose ranging from 100 to 125 mg/dL or an HbA1c between 5.7% and 6.4% but not already categorized as “diabetes.” Those with fasting blood glucose values <100 mg/dL or HbA1c <5.7%, and who were not under the “diabetes” or “prediabetes” designations, were categorized into the “normal” range.
7) Hypercholesterolemia-related variables
For those fasting >8 hours, being on cholesterol-lowering medication, or having a total cholesterol level ≥240 mg/dL were placed in the hypercholesterolemia category. Total cholesterol, triglyceride, and low-density lipoprotein (LDL) cholesterol levels were measured directly.
8) Covariates
Several covariates that may confound the association between the STOP-Bang score and glaucoma or IOP were considered. These covariates included age [15], sex [16], current smoking status [17], and presence of diabetes [18] based on a comprehensive literature review of glaucoma risk factors.
4. Statistical Analyses
All statistical analyses were performed using Stata/MP ver. 17.0 (Stata Corp., College Station, TX, USA). Descriptive statistics such as means, standard deviations, and proportions were calculated to summarize the characteristics of the study population. The chi-square test or t-test was used to assess the differences between groups, as appropriate.
Multiple regression analyses were conducted to examine the associations among the STOP-Bang score, IOP, and glaucoma. Three models were fitted. Model 1 included the STOP-Bang score as the independent variable and glaucoma or IOP as the dependent variable in the univariate model. Model 2 included the STOP-Bang score as an independent variable and glaucoma or IOP as the dependent variable as the dependent variable, with adjustments for age and sex. Model 3 included additional covariates, diabetes, and current smoking status, which are known to be high-risk factors for glaucoma, to assess the potential confounding effects.
The statistical significance level was set at P<0.05, and all reported P-values were two-sided. Results are presented as odds ratios (OR), regression coefficients (β), or their corresponding 95% confidence intervals (CI), as appropriate.
RESULTS
The study population comprised 9,495 individuals who had information from the STOP-Bang questionnaire and data on glaucoma prevalence and IOP from the eighth KNHANES (Figure 1). A total of 754 individuals were excluded for the following reasons: age ≥80 years (N=424), missing data on current smoking status (N=8), and missing data on the prevalence of comorbidities (diabetes and hypercholesterolemia) (N=322). The remaining 8,741 were included in the glaucoma prevalence analysis (Table 1). For more accurate analyses of IOP, an additional exclusion criterion of eye drop use to treat glaucoma (N=114) was applied to the subgroup analysis of IOP, leaving 8,627 individuals for the IOP analysis (Supplement 1).

Flowchart of the study participants. OSA, obstructive sleep apnea; IOP, intraocular pressure; KNHANES, Korea National Health and Nutrition Examination Survey.
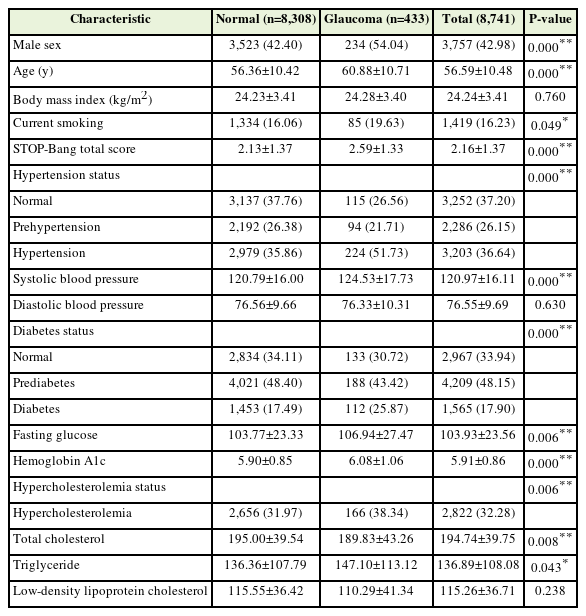
Table 1 presents a comparison of the demographics and information regarding OSA, glaucoma, and comorbidities of the normal and glaucomatous groups. Supplement 1 presents the results comparing the variables related to demographics, OSA, glaucoma, and comorbidities between the normal and glaucoma groups, with the further exclusion of individuals using eye drops to treat glaucoma. Similar patterns of differences in the variable characteristics between the normal and glaucoma groups were noted, except for the measured data related to hypercholesterolemia (Table 1, Supplement 1). Age, the STOP-Bang score, prevalence of male sex, hypertension, diabetes, hypercholesterolemia, and average SBP were significantly higher in the glaucoma than in the normal group. Triglyceride levels was significantly higher in the analysis of glaucoma prevalence (P=0.043) (Table 1), whereas it was not statistically significant in the subgroup analysis for IOP (P=0.055) (Supplement 1). BMI, average diastolic blood pressure, and LDL cholesterol levels did not differ significantly between the normal and glaucoma groups.
1. STOP-Bang Score and Glaucoma Prevalence
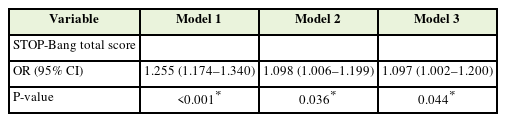
Table 2 shows the results of logistic regression within the target population for the analysis of glaucoma prevalence (n=8,741). Model 1 presents the univariate analysis results, indicating that each unit increase in the STOP-Bang score was associated with a statistically significant increase in the OR for glaucoma (OR, 1.255; 95% CI, 1.174–1.340; P<0.001). Model 2, adjusted for age and sex only, continued to show that a higher STOP-Bang score and was associated with a statistically significant increase in the OR for glaucoma (OR, 1.098; 95% CI, 1.006–1.199; P=0.036). Model 3, which further considered confounding variables, including current smoking status and prevalence of diabetes, as well as age and sex, continues to demonstrate that each unit increase in the STOP-Bang score is associated with a statistically significant increase in the OR of glaucoma (OR, 1.097; 95% CI, 1.002–1.200; P=0.044).
2. STOP-Bang Score and Intraocular Pressure
Table 3 shows the results of linear regressions within the target population for the subgroup analysis of IOP (n=8,627). Model 1 presents the univariate analysis results, indicating a positive value of the beta coefficient, meaning that each unit increase in the STOP-Bang score is associated with a statistically significant increase in IOP (β=0.065; 95% CI, 0.030–0.101; P<0.001). Model 2, adjusted for age and sex only, also showed that a higher STOP-Bang score is associated with a statistically significant increase in IOP (β=0.215; 95% CI, 0.170–0.261; P<0.001). Model 3, which further considers confounding variables including current smoking status and prevalence of diabetes, in addition to age and sex, continued to demonstrate that each unit increase in the STOP-Bang score is associated with a statistically significant increase in IOP (β=0.171; 95% CI, 0.125–0.218; P<0.001).
DISCUSSION
In this study, we investigated the potential association between OSA risk, as measured using the STOP-Bang score, and the prevalence of glaucoma and IOP in a Korean population. Our findings revealed that a higher STOP-Bang score, which denotes a higher likelihood and severity of OSA, was associated with a higher prevalence of glaucoma and increased IOP. These findings, which build on previous research hinting at a link between OSA and glaucoma, reinforce the notion that OSA is indeed associated with the onset and progression of glaucoma as well as systemic comorbidities such as cardiovascular disease and metabolic disorders.
When comparing the glaucoma and normal groups, significant differences were noted related to age, male sex, hypertension, diabetes, hypercholesterolemia, and higher SBP, all of which are recognized risk factors for glaucoma. This finding aligns with the findings of previous studies, including the study by McMonnies [15], which identified risk factors for glaucoma, including old age, male sex, family history of glaucoma, the African American or Asian race compared to the Caucasian race, myopia, and hypertension.
Considering the components of STOP-Bang score, where a higher score indicates greater OSA risk and severity, several objective assessment items—such as hypertension, a high BMI, age ≥50 years, and male sex—overlapped with glaucoma risk factors. This finding is consistent with that of a previous study exploring the association between OSA and glaucoma, attributing it to shared vascular or mechanical mechanisms and risk factors [17].
However, considering the shared risk and severity factors of OSA and glaucoma, it is important to recognize that their complex relationships are not yet fully understood. Contrary to initial expectations, no significant differences in BMI, DBP, and LDL cholesterol levels were observed between the normal and glaucoma groups.
Research using the Comprehensive All of Us database suggests that metabolic syndrome may present a higher risk of glaucoma than BMI alone [19]. Furthermore, the role of low nocturnal diastolic ocular perfusion pressure [20] or dipping patterns in diurnal variation [21] as important predictive factors for visual field progression in NTG further complicates the relationships among blood pressure, IOP, and glaucoma. A recent meta-analysis found an significant association between glaucoma and high total cholesterol and low HDL cholesterol levels but not high LDL cholesterol levels [22], potentially due to variations in glaucoma types or study designs, including the use of lipid-lowering medications. The absence of notable differences in BMI, DBP, and LDL cholesterol levels between the normal and glaucoma groups in our study is consistent with these findings. Thus, the finding suggest that although OSA and glaucoma risk factors overlap, careful attention is needed to interpret their dynamics.
Notably, our results underscore that the association between OSA and glaucoma prevalence persists even after adjusting for known glaucoma risk factors such as age, sex, current smoking status, and diabetes, aligning with studies exploring the association between OSA and glaucoma. A recent systematic review and meta-analysis of 48 observational and cross-sectional studies revealed that OSA is associated with a high risk of glaucoma (OR, 3.66) [23]. Furthermore, Lin et al. [24] showed in a Taiwanese retrospective matched-cohort study that the hazard ratio for open-angle glaucoma within a 5-year period for participants with OSA was 1.67 times higher than those for control participants.
These findings contribute to a growing body of evidence suggesting that the pathophysiological mechanisms underlying the relationship between OSA and glaucoma could extend beyond shared risk factors, warranting further research. Although research is ongoing, multiple pathophysiological mechanisms such as hypoxia, vascular issues, and mechanical factors are suggested to contribute to glaucoma in OSA patients [1]. Prolonged episodes of apnea and hypopnea in patients with OSA are believed to cause oxidative stress, inflammation, sympathetic overdrive, and endothelial cell damage, ultimately damaging the optic nerve head, retinal ganglion cells, and their axons, resulting in glaucoma [25].
Furthermore, our study demonstrated a significant and positive relationship between the STOP-Bang score and IOP, which is a well-established risk factor for glaucoma. This association could stem from OSA-related intermittent hypoxemia and arousal, potentially altering the balance between aqueous humor production and outflow, leading to increased IOP [24]. Some studies have shown that continuous positive airway pressure therapy, a well-known treatment for OSA, can raise IOP [26], which could further augment the risk of glaucoma in the affected individuals. Most previous studies examining the relationship between OSA and glaucoma have primarily focused on glaucoma prevalence or incidence, with less attention paid to its direct correlation with IOP. However, given that elevated IOP is the most well-known risk factor for glaucoma, the specific nature and implications of the relationship between OSA, IOP, and glaucoma require further exploration, ideally in longitudinal studies, to establish causality and understand the underlying pathophysiological mechanisms.
The strength of our study is that it is the first study to examine the relationship between the STOP-Bang score and both glaucoma and IOP in a large Korean population using data from the KNHANES. Our results are consistent with those of numerous prospective and retrospective large cohort studies as well as cross-sectional studies that have shown positive hazard ratios of glaucoma in patients with OSA patients [23].
Furthermore, our findings suggest that the STOP-Bang score may offer potential utility as a preliminary and non-invasive screening tool for identifying individuals who might be at risk of developing glaucoma based on the association between the STOP-Bang score and glaucoma or IOP. A STOP-Bang score ≥3 exhibits significant sensitivity (>90%) and substantial discriminative power in excluding moderate-to-severe and severe OSA, with corresponding negative predictive values of 77% and 91%, respectively [27]. Identifying individuals at increased risk of OSA raises the prospect of early intervention to alleviate the burden of irreversible glaucoma. Considering the simplicity of administering the STOP-Bang questionnaire, our results have implications for clinical practice, potentially leading to its integration into routine health check-ups. This integration might provide a pathway for higher-risk patients with OSA to consider precautionary measures, such as regular IOP checks or fundus examinations, contributing to the mitigation of the risk of developing or exacerbating glaucoma.
Nevertheless, our study has certain limitations that warrant acknowledgment. The cross-sectional design of this study inhibited our ability to infer a direct cause-effect relationship between the STOP-Bang score and glaucoma risk. Consequently, additional research, particularly longitudinal studies, is necessary to validate our findings and to elucidate the temporal relationship between OSA and glaucoma. Additionally, in the context of diverse glaucoma forms, this study refrained from delineating specific subgroups within the glaucomatous spectrum. Instead, it comprehensively addressed the overarching correlation between glaucoma and OSA. Consequently, additional research is required to elucidate the nuanced association between OSA and individual glaucoma subtypes. Moreover, while the STOP-Bang score is a reliable and validated tool for assessing OSA risk and severity, it cannot replace formal polysomnography in diagnosing OSA. Therefore, future studies utilizing both polysomnography results and STOP-Bang scores are essential to elucidate the relationship between OSA and glaucoma. Finally, while the adjusted OR of model 3 remained statistically significant, it is crucial to note that the association between the STOP-Bang score and glaucoma prevalence may be influenced by the robust relationship between age and glaucoma. Hence, further research in different settings, with varying samples or study designs, is warranted.
In conclusion, our study findings suggest an association between OSA risk, assessed using the STOP-Bang score, and increased prevalence of glaucoma and IOP values, even after adjusting for potential confounding variables. Thus, it is imperative for clinicians to consider early and frequent glaucoma screening in individuals at a higher risk for OSA, highlighting the potential role of the STOP-Bang score as a simple and noninvasive tool for assessing glaucoma risk in patients with OSA.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.23.0162.
Demographics of the study population for the sub-group analysis of intraocular pressure