Correlation of Arterial Stiffness and Bone Mineral Density by Measuring Brachial-Ankle Pulse Wave Velocity in Healthy Korean Women
Article information
Abstract
Background
An association between arterial stiffness and osteoporosis has previously been reported. Therefore, we investigated the relationship between arterial stiffness, measured by brachial-ankle pulse wave velocity, and bone mineral density in a sample of healthy women undergoing routine medical checkup.
Methods
We retrospectively reviewed the medical charts of 135 women who had visited the Health Promotion Center (between May 2009 and December 2012). Brachial-ankle pulse wave velocity was measured using an automatic wave analyzer. Bone mineral density of the lumbar spine (L1-L4) and femur was measured by dual-energy X-ray absorptiometry. Metabolic syndrome was defined according to National Cholesterol Education Program-Adult Treatment Panel III criteria, using body mass index >25 kg/m2 instead of waist circumference >88.9 cm.
Results
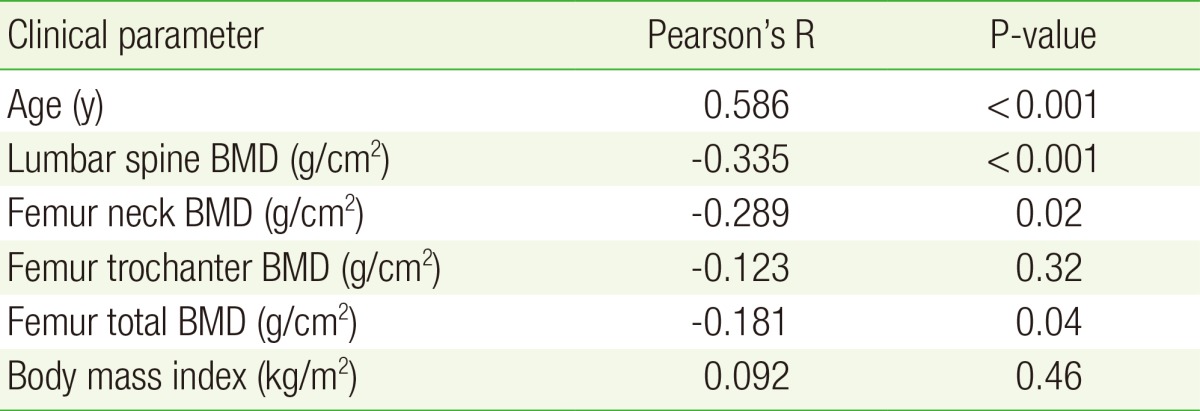
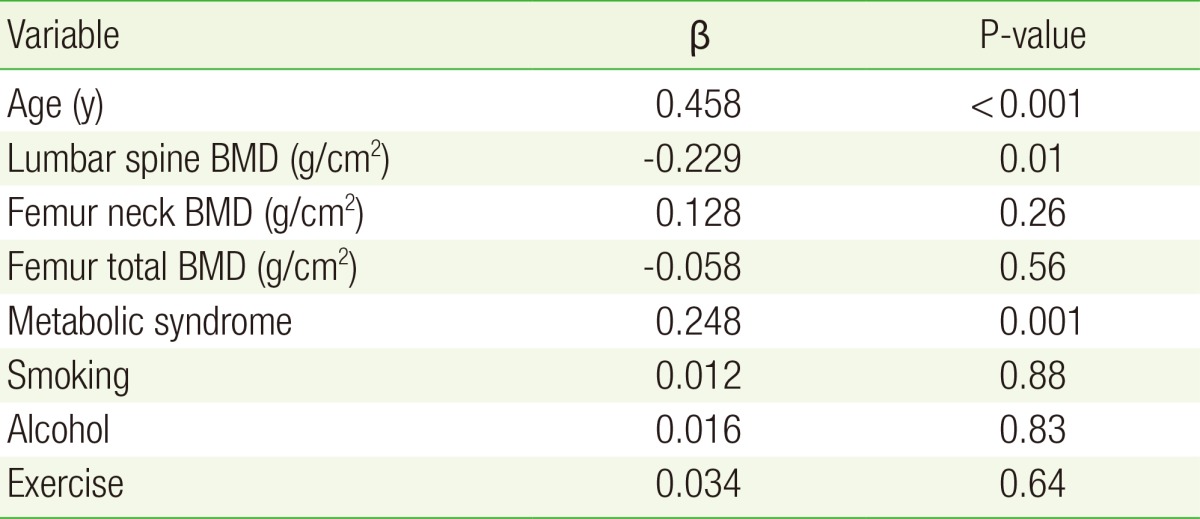
Pearson's correlation analysis revealed significant inverse relationships between pulse wave velocity and bone mineral density of the lumbar spine (r=-0.335, P<0.001), femur neck (r=-0.335, P<0.001), and total femur (r=-0.181, P=0.04). Pulse wave velocity showed the strongest association with age (r=0.586, P<0.001). Multiple regression analysis identified an independent relationship between pulse wave velocity and lumbar spine bone mineral density in women after adjusting for age, metabolic syndrome, body mass index, smoking status, alcohol intake, and exercise (r=-0.229, P=0.01).
Conclusion
This study confirmed an association between arterial stiffness and bone mineral density in women.
INTRODUCTION
Cardiovascular disease (CVD) and osteoporosis are two of the major causes of death and illness in the elderly. Some researchers have found that these conditions, the risk of both of which increases with age, are independently correlated with age. In particular, a correlation between bone mineral density (BMD) and death from CVD has been observed among postmenopausal women.12) Lower BMD has been associated with a higher risk of coronary artery disease34) and several studies report bone loss accompanied by vascular calcification.567)
Researchers have also investigated the correlation between arterial stiffness and osteoporosis.8910) Although there are several measures of arterial stiffness, including central blood pressure (BP) and the augmentation index (AI), pulse wave velocity (PWV) is widely used as it is convenient, noninvasive, and inexpensive. The measurement of arterial stiffness may help predict the risk of cardiovascular diseases and determine the efficacy of treatment for these conditions.
In clinical practice, brachial-ankle pulse wave velocity (ba-PWV), which records the pulse wave using an oscillometry sensor on brachial-ankle BP cuffs, is frequently used because it does not require sophisticated technology or exposure of the inguinal area. Recent reports indicate that ba-PWV is correlated with carotid-femoral PWV;1112) although the former of the two methods has been less extensively researched, it was found to be useful in predicting CVD mortality among patients with cardiovascular diseases, renal failure, or heart failure.131415) Vascular calcification can cause arterial stiffness, and both PWV and vascular calcification may reflect atherosclerosis.
The correlation between osteoporosis and CVD or CVD risk factors, such as atherosclerosis, arterial stiffness, and vascular calcification, is still controversial and findings concerning this correlation are not consistent with one another. Here, we aimed to use ba-PWV to analyze the relationship between arterial stiffness and BMD of both, the lumbar spine (L-spine) and hip (femur) joints in healthy women undergoing routine medical checkup.
METHODS
1. Subjects
Medical charts were analyzed retrospectively for 135 women who had undergone routine medical checkup at the Health Promotion Center (between May 2009 and December 2012). We reviewed the patient-doctor consultation records for information about each patient's lifestyle habits, medical history, previous medication, smoking status, and alcohol intake. Patients were asked to take off their outer garment before their height and weight were measured on a single height and weight scale; body mass index (BMI) was calculated on the basis of weight (kg) and height (m). For all patients, the basic regular checkup comprised a blood test, ba-PWV, and dual-energy X-ray absorptiometry (L-spine and femur). The blood test was performed after a ≥12-hour fast and BP was measured using an electronic manometer on the right arm after a ≥10-minute rest. Women who had a medical history of hypertension, diabetes, hyperlipidemia, chronic renal failure, thyroid disease, parathyroid disease, or rheumatoid arthritis, who had used bisphosphonate or steroid medications, or who were diagnosed with cancer less than five years prior were excluded from the study.
2. Clinical Measurements
BMD was measured in the L-spine (L1-L4), femur neck, and total femur using dual-energy X-ray absorptiometry (Lunar Prodigy Advance; GE Healthcare Technologies, Madison, WI, USA). The results were presented in g/cm2.
Patients were asked to rest in the supine position for 10 minutes before PWV was measured using an automatic waveform analyzer (VP-1000; Clin Co., Komaki, Japan). Oscillometry was used to measure BP in both arms (posterior tibial artery) and ankles (brachial artery); the ankle-brachial index was calculated automatically. Right and left ba-PWVs were measured simultaneously, and the mean of both ba-PWVs was used for all analyses.
Patients were categorized into groups on the basis of their CVD risk factors: smoking group (current smokers) versus nonsmoking group (including former smokers); drinking group (patients consuming ≥1.5 bottles of alcohol per week) versus nondrinking group (patients drinking <1.5 bottles of alcohol per week); exercise group (patients exercising for ≥30 minutes per day or ≥3 times a week) versus no-exercise group (patients exercising for <30 minutes per day or ≤3 times a week).
We defined metabolic syndrome in accordance with the guidelines of the 2000 World Health Organization Recommendation for Obesity Standards in Asian-Pacific Regions and the National Cholesterol Education Program-Adult Treatment Panel III,16) referring to the patient blood test results and replacing the waist circumference criteria (>88.9 cm for women) with BMI >25 kg/m2. Those patients satisfying ≥3 of the following diagnostic criteria were considered to have metabolic syndrome: (1) BMI ≥25 kg/m2; (2) fasting triglycerides ≥150 mg/dL; (3) fasting high density lipoprotein cholesterol <50 mg/dL; (4) fasting blood glucose ≥100 mg/dL; and (5) BP ≥130/85 mm Hg.
3. Statistical Analysis
All variables were presented as mean±standard deviation. Independent t-tests were used to analyze variation in ba-PWV by metabolic syndrome status, alcohol intake, smoking status, and exercise habits. Pearson correlation analysis was performed to evaluate the relationship between age, BMI, and regional BMD and ba-PWV.
Multiple regression analysis was performed with ba-PWV as the dependent variable; all variables that showed statistically significant relationships with ba-PWV upon independent t-test and Pearson correlation analysis were included as covariables. Variables that were not significantly related to ba-PWV (P>0.05) but were considered to act clinically as confounding variables (alcohol intake, smoking status, exercise, and metabolic syndrome) were also included as covariables. The significance level was set at P<0.05 and all the statistical analyses were conducted using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Subject Characteristics
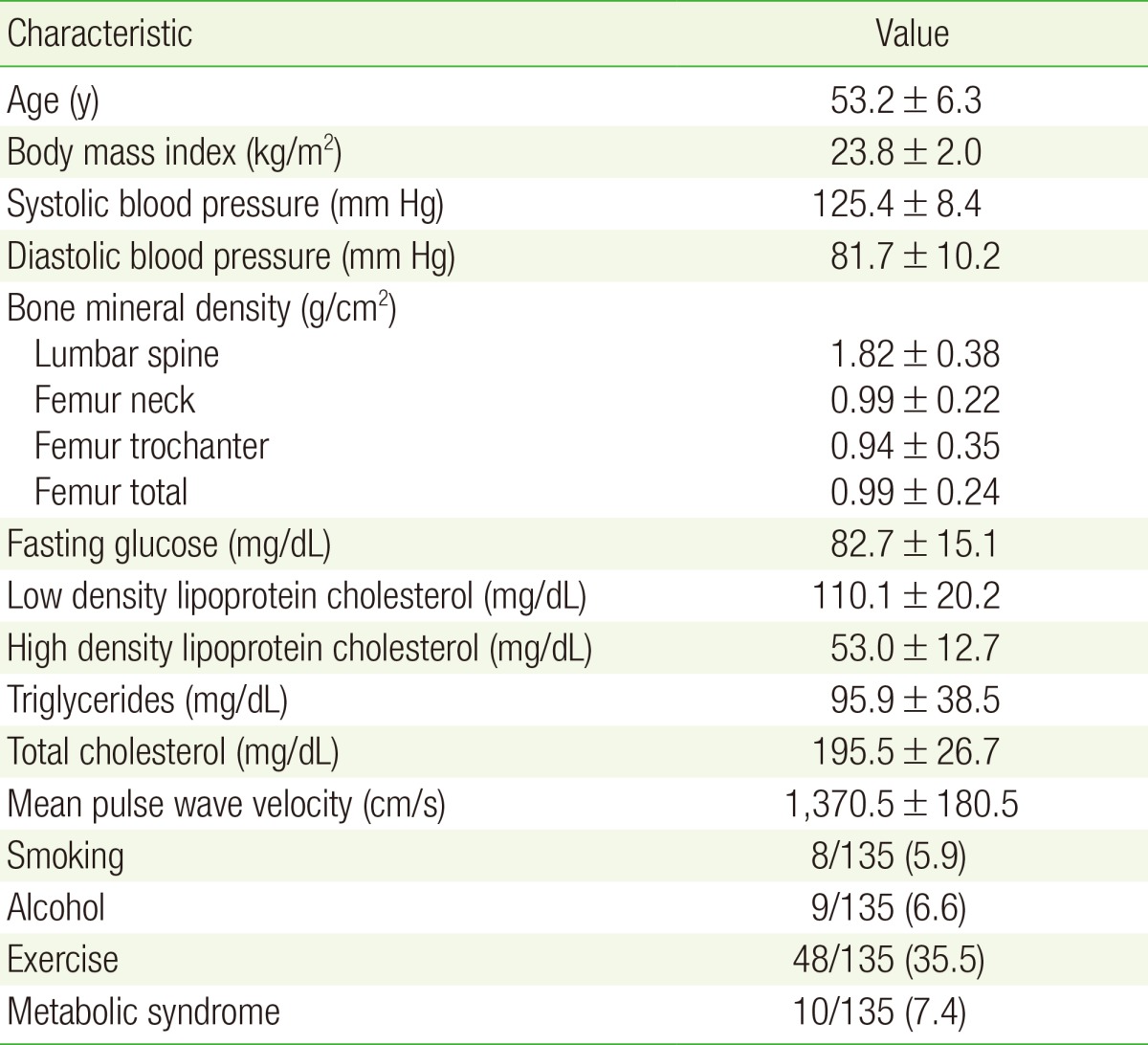
The 135 women in this study had an average age of 53 years and mean BMI of 23 kg/m2. The mean ba-PWV was 1,370.5±180.5 cm/s; mean values of other clinical variables and CVD risk factors are shown in Table 1.
2. Univariate Analysis
Independent t-tests revealed no significant differences in ba-PWV among women on the basis of their alcohol intake, smoking behavior, or exercise status (P=0.14, P=0.65, and P=0.42, respectively). In contrast, the metabolic syndrome group showed significantly higher ba-PWV values than the nonmetabolic syndrome group (1,349.16±97.62, P<0.01) (Table was not shown).
Pearson correlation analysis revealed a significant negative correlation between ba-PWV and L-spine BMD, femur neck BMD, and total femur BMD (as shown in Table 2). In contrast, no correlation was found between trochanter femur BMD and ba-PWV, nor between BMI and ba-PWV (Table 2). Age was the factor most strongly correlated to ba-PWV (r=0.586, P<0.001).
3. Multivariable Analysis
In addition to statistically significant outcome variables from the univariate analysis, potential clinically confounding variables (alcohol intake, smoking status, and exercise) were used as covariables in the regression analysis. Independent of these factors, ba-PWV was shown to be significantly and negatively correlated with L-spine BMD but was not significantly associated with BMD in the femoral neck or total femur (Table 3). Age was the factor most strongly predictive of ba-PWV; a relationship was also observed between metabolic syndrome and ba-PWV on multivariate analysis (Table 3).
DISCUSSION
This study evaluated the correlation between arterial stiffness and BMD in healthy women undergoing regular medical check-up. Multivariate analysis identified an independent, significant, and negative correlation between ba-PWV, a measure of vascular stiffness, and lumbar spine BMD. Metabolic syndrome was also significantly correlated with ba-PWV, while age was the variable most strongly correlated with PWV.
The correlation between BMD and vascular stiffness has not hitherto been clearly established by epidemiological or clinical studies including the study by the Japanese researchers. Age, smoking, hypertension, diabetes, hyperlipidemia, renal failure, physical activity, and menopause are risk factors for both conditions. In addition, inflammatory factors, such as oxidized low density lipoprotein, osteopontin, and osteoprotegerin, as well as other substances that affect bone health, such as vitamin D and estrogen, are expected to influence the relationship between BMD and vascular stiffness.17181920)
A correlation between osteoporosis and increased ba-PWV has been observed during a study to determine the association between lumbar BMD and ba-PWV in postmenopausal women. 9) As in this study, a significant negative correlation was also found between lumbar BMD and vascular stiffness-measured by both AI and PWV-among obese African-American women (P=0.01); though the relationship was only marginally significant, vascular stiffness was also inversely proportional to the femoral neck BMD (P=0.05) and total hip BMD (P=0.06).10)
Frost et al.21) found that PWV was significantly inversely proportional to femoral BMD but was not significantly correlated with lumbar BMD among postmenopausal women. This result is probably due to osteophyte, osteosclerosis, osteophytosis, or vascular calcification. Women may experience a decrease in spongy bone postmenopausally; since spongy bone distribution varies between the L-spine and the femoral neck, a reduction in BMD may first be noticed in the spine and only later in cortical and spongy bone, such that lower femoral BMD is not evident until the age of 70 years. One study of a Japanese population also found that lumbar BMD began to decrease in women in their 50s, around the time of menopause.22) The population in this study comprised a group of women aged 53 years, on average, who were experiencing postmenopausal bone loss; therefore, a significant correlation was observed between lumbar BMD and PWV. The fact that degenerative arthritis and vascular calcification are more likely to occur with advancing age may explain the weak correlation with lumbar BMD and stronger correlation with femoral BMD in older patient cohorts.
In this study, a significant correlation was observed between features of metabolic syndrome and ba-PWV. Consistent with this finding, multiple Asian studies have reported that metabolic syndrome leads to significantly higher PWV, especially among women.2324) The results of our study should be interpreted with caution because BMI, instead of waist circumference, was used to classify metabolic syndrome. However, PWV, which is a measure for arterial stiffness and atherosclerosis, may increase due to BP, diabetes, and hyperlipidemia and is probably correlated with metabolic syndrome, as found in previous research. Further research should be conducted because ba-PWV has rarely been researched in healthy adults without hypertension, diabetes, or hyperlipidemia, because BP can act as a confounding factor, and because no definite conclusion has been drawn about correlation with metabolic syndrome in which insulin resistance and body fat are regarded as important factors.
With age comes the development of arterial stiffness and an often remarkable rise in systolic BP. Here, the finding that ba-PWV was proportional to age is consistent with the fact that arterial stiffness is proportional to age.
We acknowledge some limitations to this study. Subject data was obtained from chart review and was limited with respect to menopausal status. As the decrease in estrogen production after menopause can contribute to the development of atherosclerosis and osteoporosis, menopausal status could have been a confounding factor in our analysis. In addition, osteoporosis can be caused by calcium and vitamin D deficiency and some researchers have reported that calcium and vitamin D levels are correlated with arterial stiffness;25) however, no consideration was given to these nutrients in this study.
In conclusion, our analysis demonstrated that lumbar spine BMD was independently and significantly correlated with ba-PWV in women after correction for age, metabolic syndrome, alcohol intake, and exercise status (r=-0.229, P=0.01). Further epidemiological and clinical research should be conducted to investigate the correlation between BMD and arterial stiffness in men and women.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.