Relationship between Changes in Fatigue and Exercise by Follow-Up Period
Article information
Abstract
Background
Fatigue is one of the most common presenting symptoms in primary care in Korea. In this study, we aimed to determine the effect of exercise intervention on the severity of fatigue of unknown medical cause during a period of follow-up.
Methods
We used the data collected from an outpatient fatigue clinic in Seoul National University Bundang Hospital. The study was conducted from March 3, 2010 to May 31, 2014. We measured the body mass index of each patient and evaluated variables including lifestyle factors (smoking, alcohol consumption, and regular exercise), quality of sleep, anxiety, depression, stress severity, and fatigue severity using questionnaires. A total of 152 participants who completed questionnaires to determine changes in fatigue severity and the effect of exercise for each period were evaluated. We used univariate analysis to verify possible factors related to fatigue and then conducted multivariate analysis using these factors and the literature.
Results
Of 130 patients with the complaint of chronic fatigue for over 6 months, over 90 percent reported moderate or severe fatigue on the Fatigue Severity Scale and Brief Fatigue Inventory questionnaires. The fatigue severity scores decreased and fatigue improved over time. The amount of exercise was increased in the first month, but decreased afterwards.
Conclusion
There was no significant relationship between changes in the amount of exercise and fatigue severity in each follow-up period. Randomized controlled trials and a cohort study with a more detailed exercise protocol in an outpatient setting are needed in the future.
INTRODUCTION
Fatigue is a symptom that is commonly seen in primary care. A study of 1,648 patients over 18 years of age in eight local primary care institutions reported that 21.1% complained of persistent fatigue for six months or less, and 8.4% reported chronic fatigue for more than 6 months.1) Chronic fatigue prevention and treatment is necessary because it interferes with activities of daily living and exerts a major impact on quality of life.2) However, there is no standardized questionnaire for fatigue severity since the level of perceived fatigue is different for each person, and the physical, mental, and socioeconomic causes are diverse.3) For these reasons, fatigue is difficult to study.
The causes of chronic fatigue in the primary health care study in Korea 2005 were mental factors, around 46% (stress, depression, adjustment disorders, anxiety disorders, sleep disorders, hwa-byung, alcoholism, and panic disorder), organic factors, approximately 34% (uncontrolled diabetes, asthma, chronic hepatitis, tuberculosis, thyroid function abnormality, iron deficiency anemia, fatty liver disease, sleep apnea, chronic obstructive pulmonary disease, and herniated intervertebral disc), and unknown factors, about 20%.1) It has been reported that factors related to fatigue include age, gender, body mass index (BMI), lifestyle (smoking, alcohol consumption, regular exercise, and caffeine intake), psychological state (stress, depression, and anxiety), marital status, social life, occupation, sleep disorder, and autonomic nervous system.145)
Most previous studies considered specific conditions known to be associated with fatigue, such as illnesses (cancer,6) ischemic heart disease, 7) systemic lupus erythematosus,8) multiple sclerosis,9) etc.), and sleep disorders,10) anxiety, or depression.11) However, there are few studies of chronic fatigue without known organic cause in the primary care setting. In a study with a primary care setting, negative life events and lack of physical fitness were related with new onset fatigue.12) In another study, chronic fatigue symptoms treated with counseling and graded exercise for one year improved, compared to a usual care control group, but the improvement was not significant.13) There have been randomized controlled trials on the effects of aquatic exercise in women who complained of joint pain,14) and the effect of walking exercise intensity on fatigue and blood lipid changes in middle-aged women, 15) but these studies were limited by being conducted on a small number of specific conditions, and did not consider previous known factors that can cause fatigue.
The present study is limited to fatigue patients without an organic cause, such as malignancy, uncontrolled diabetes, asthma, chronic hepatitis, tuberculosis, ischemic heart disease, thyroid dysfunction, or iron deficiency anemia. Lifestyle and physical factors that exacerbate fatigue, such as obesity, smoking, drinking, and sleep disorders, and intermittent medication for mental factors related to fatigue, such as anxiety, depression, stress, and sleep disorders, were evaluated in this study. We assessed the effect of changes in the amount of exercise after initiating treatment interventions on changes in the severity of fatigue according to follow-up periods over time.
METHODS
1. Study Population
This study was conducted on patients who visited a fatigue clinic in the department of family medicine, Seoul National University Bundang Hospital, from March 3, 2010 to May 31, 2014. We excluded 145 patients who only visited once, 38 who did not complete the exercise questionnaire after the first visit, and 4 who did not return to the clinic within 6 months. Finally, we analyzed 152 patients for whom exercise and fatigue changes could be determined for each follow-up period.
2. Study Procedures
We recorded gender, age, and BMI at the first visit, and smoking, drinking, exercise, fatigue, sleep quality, anxiety, depression, and stress level were investigated through a self-reporting survey. Follow-up during the study period was performed at 3 time points: within 1 month after the first visit, within 1 to 3 months, and within 3 to 6 months.
1) Age, body mass index, social behaviors
Age and BMI were classified as continuous variables, and smoking was counted as a categorical variable, reported as 'never smoked,' 'exsmoker,' or 'current smoker.' Drinking was categorized as 'none or average drinker' or 'current drinker.' Risk drinking was defined as over 40 g of alcohol daily for men and 20 g for women using the World Health Organization criteria.16)
2) Fatigue
Evaluation of fatigue was conducted using the Fatigue Severity Scale (FSS) and the Brief Fatigue Inventory (BFI). The FSS is widely used to examine the effects of fatigue, and has high reproducibility among the most common fatigue surveys.3) The validity of the Korean version of FSS questionnaire was demonstrated.17) FSS assesses the status of fatigue for the prior week using 9 questions graded from 1 to 7 points for each item, and the average is calculated. A higher score means a greater degree of fatigue. We classified the high fatigue group as having a score of more than 4.5 points, moderate as 3 to 4.5 points, and mild as less than 3 points, with reference to the literature on FSS.317)
The BFI was developed to rapidly measure the degree of fatigue in cancer patients,18) and the reliability and validity of the Korean version was has been reported.19) The usefulness of the BFI for evaluating fatigue severity and associated factors in the general population has been documented in community research.20) The BFI consists of 9 questions on fatigue and its interference with daily life in the prior 24 hours, and is scored from 0 to 10 points for each question, plus the total. An increase in the average score means that the severity of fatigue is increased. The first 3 questions ask the subject to describe the current level of fatigue, the usual level of fatigue during the previous 24 hours, and the maximum level of fatigue during the previous 24 hours. Unlike the next 6 questions about the relationship between the average level of functioning and mood, the first 3 questions measure the severity of fatigue, and we used these to calculate the change in level of fatigue in this study.20) We classified the severe fatigue group as those who scored at least 7 points by reference to the literature, moderate as 4 to less than 7 points, and mild as less than 4 points.19)
3) Exercise amount
The type of exercise reported in medical records and questionnaires was converted into dynamic physical activity according to metabolic equivalents (METs). We converted values to minutes of running time for workout activities performed continuously for at least 10 minutes at a time in one week, and then summed the values to classify a 'low exercise group (600 MET-min/wk or less),' a 'moderate exercise group (600 to 1,500 MET-min/wk or less),' and a 'high exercise group (1,500 MET-min/wk or more).'21)
4) Sleep quality
Quality of sleep was evaluated by the Pittsburgh Sleep Quality Index (PSQI), which was developed to rate the quality of sleep and identify sleep disorders. The PSQI assesses subjective sleep quality, latency, duration, habitual sleep behavior, sleep disturbance, sleep drug use, and daytime dysfunction. Each item is scored as 0 to 3 points; we classified 'good sleep quality' as a score of 5 points or less and 'bad sleep quality' as 6 points or more.22)
5) Anxiety and depression
Anxiety and depression were evaluated using the Korean version of the short Goldberg Anxiety and Depression Scale. The Goldberg scale has been proven to identify anxiety and depression, and the Korean version has been validated.23) It consists of 18 questions, including 4 for major symptoms and 5 for minor symptoms for each of anxiety and depression. Each item is scored as 0 or 1 point, and the higher the total score, the more severe the anxiety and depression. We classified the 'anxious' group by a score of 5 or more points for anxiety-related questions and the 'depressed' group by a score of 5 or more points for depression-related questions.24)
6) Stress
The amount of stress was measured using the Korean version of the Brief Encounter Psychosocial Instrument (BEPSI-K). The BEPSI-K comprises 5 questions scored on a 5-point scale; the greater the total, the higher the level of stress. We allocated the subjects with a score of 2.4 or more points in the "stressed group".25)
3. Statistical Analysis
Univariate analysis was performed, with correlation analysis, t-tests, and analysis of variance comparing fatigue scores in each follow-up period and variables such as gender, age, BMI, smoking, alcohol, anxiety, depression, stress, and the quality of sleep. Multiple linear regression analysis compared changes in level of fatigue and the change in the amount of exercise in each follow-up period, including fatigue-associated disturbance variables. Statistical significance was set at a level of less than 5%. We used STATA SE ver. 12.1 (Stata Co., College Station, TX, USA) for data analysis.
RESULTS
1. Baseline Characteristics
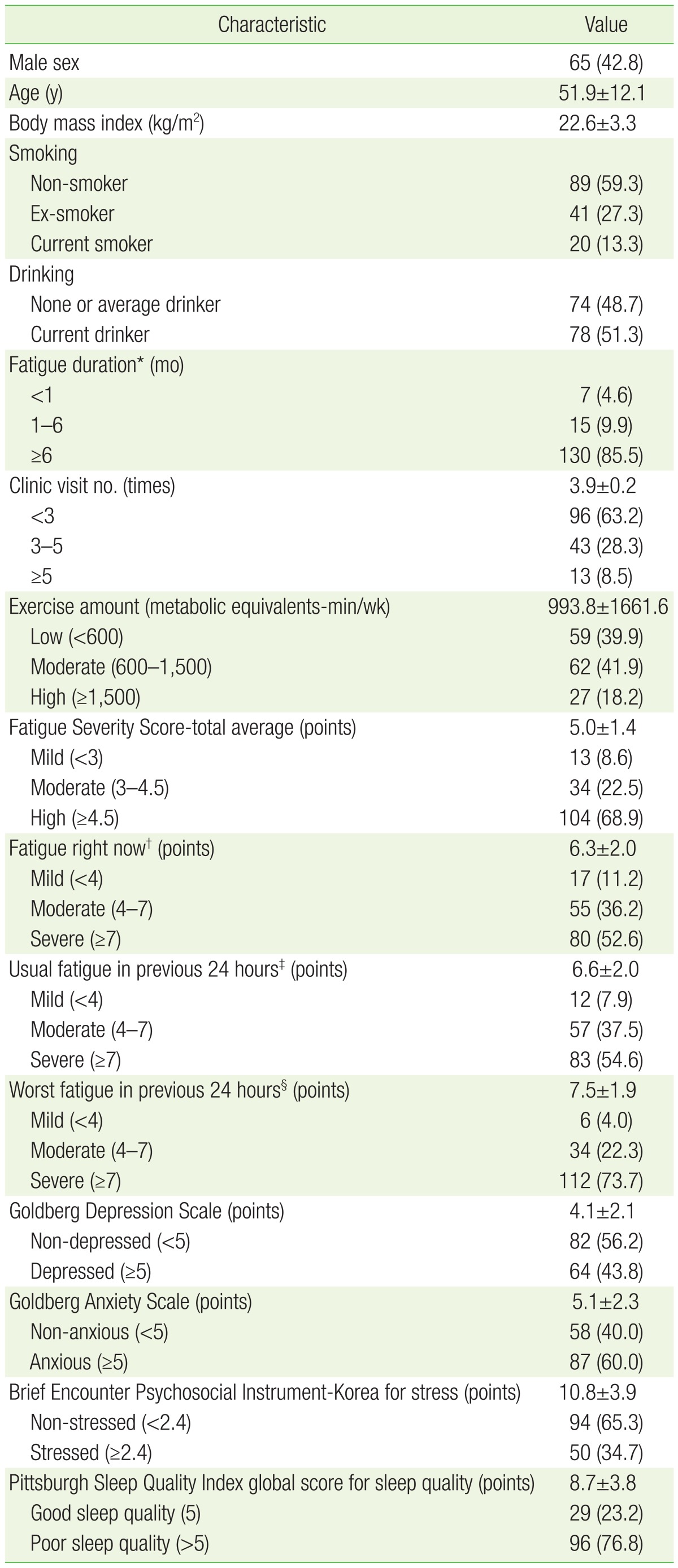
Of 152 subjects, there were more females (87) than males (65), and the mean age was 51.9 years. There were 130 non-smokers and ex-smokers (86.6%), and 74 none or average drinkers (48.7%). Those who complained of chronic fatigue for 6 months or more accounted for 130 patients (85.5%). There were 138 with moderate to severe fatigue on the FSS (91.4%), and 135 (88.8%), 140 (92.1%), and 146 (96.0%) on the first 3 questions of the BFI, respectively. In the initial survey, there were 89 (60.1%) who performed moderate or greater physical activity. There were 96 (76.8%) who complained of poor sleep quality, and 87 (60.0%) with suspected anxiety disorder. There were 96 patients who visited 3 times or less, and 13 who visited more than 5 times (Table 1).
2. Subject Numbers, Exercise Amount, and Fatigue Scale Differences in Each Follow-Up Period
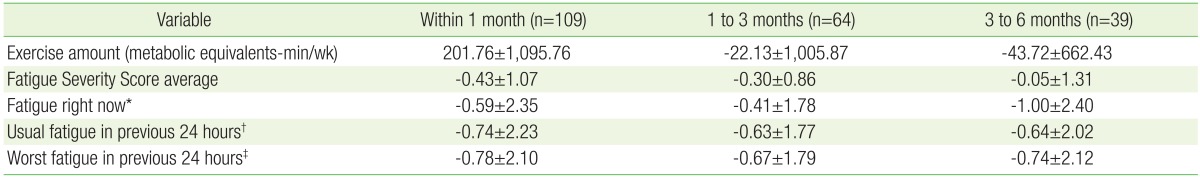
Follow-up time points were categorized as within 1 month, 1 to 3 months, and 3 to 6 months after the first fatigue clinic visit, respectively. A total of 152 cases were studied after all fatigue and exercise surveys were completed, and then the data for the 109, 64, and 39 subjects tracked during the above respective time periods were analyzed. All levels of fatigue decreased with time, but the amount of exercise only increased within the first month, and decreased thereafter (Table 2).
3. Correlation between Exercise Amount and Fatigue in Each Follow-Up Period
We conducted correlation analysis comparing changes in level of fatigue using 4 questionnaire scores and changes in exercise amount to calculate results for initial and final visit survey data in each period. There was a negative association (P=0.02) between the amount of exercise change in the period from 3 to 6 months after the first visit and the change in level of fatigue described at the time of the visit; there was also a negative association between the change in the BMI and changes in the average fatigue level for the prior 24 hours (P=0.02). Women tended to show more improvement than men in the changes in level of fatigue for the prior day (P=0.03) and for the maximum fatigue level in the prior 24 hours (P=0.01) in the period of follow-up within one month. During the same period, fatigue improvement in current drinkers tended to be less than that in none or average drinkers (P=0.01).
4. Multivariate Analysis of Fatigue Changes according to Differences in Activity in Each Follow-Up Period
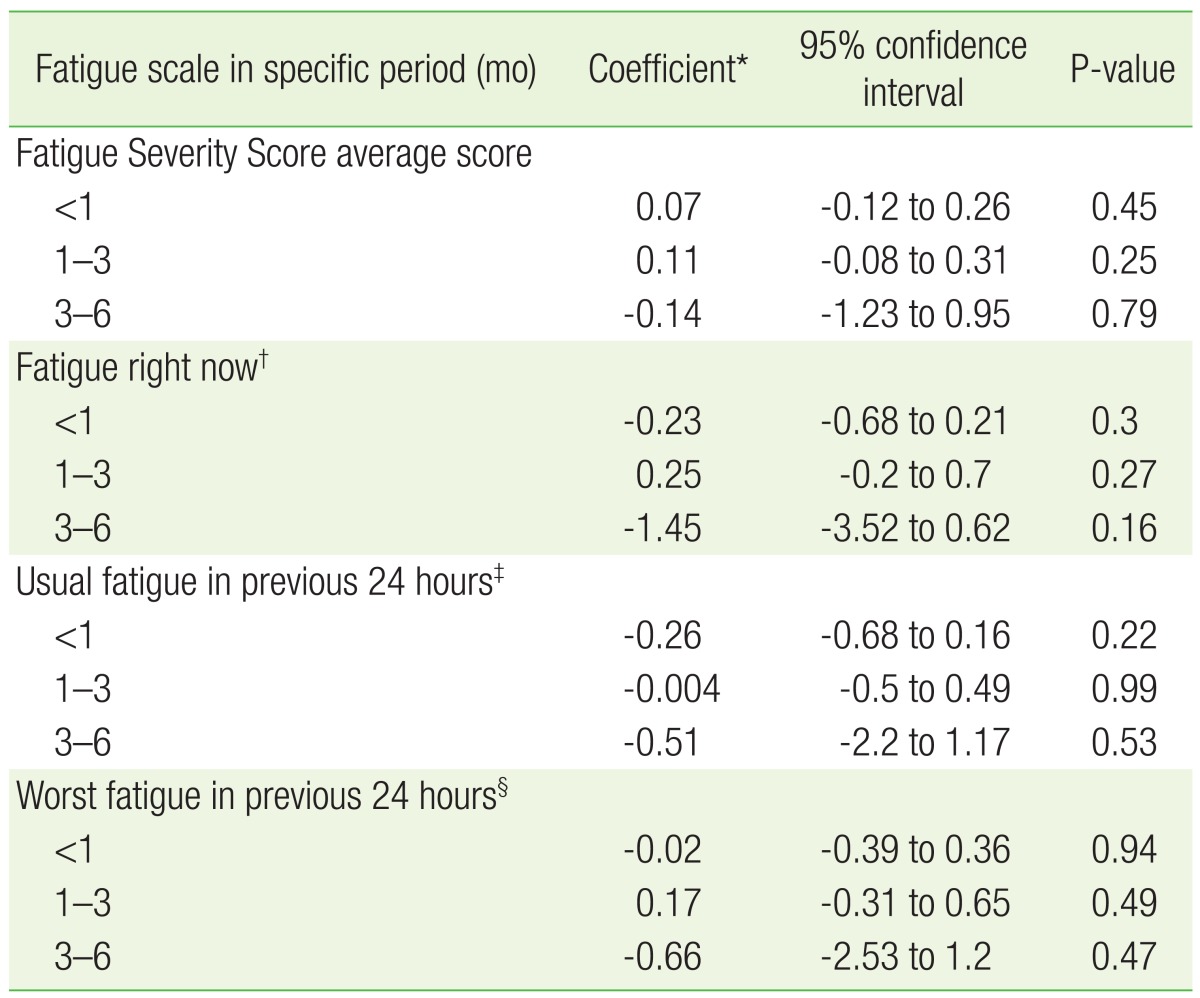
Based on the univariate analysis and the literature, we performed multiple linear regression analysis for confounding variables associated with fatigue, such as anxiety, depression, stress, quality of sleep, fatigue duration, gender, age, BMI, smoking, and alcohol consumption. The change in fatigue level in each period was calculated using the 4 different questionnaire scores at the initial and final visit of each follow-up period. The FSS was not statistically significant in any follow-up period (P-value=0.45, 0.25, 0.79 at less than 1 month, 1 to 3 months, 3 to 6 months, respectively). The response to BFI question 1 regarding fatigue changes at the time of questionnaire completion was not statistically significant (P-value=0.3, 0.27, 0.16 at less than 1 month, 1 to 3 months, 3 to 6 months, respectively); the response to BFI question 2 about average fatigue changes during the prior 24 hours (P-value=0.22, 0.99, 0.53 at less than 1 month, 1 to 3 months, 3 to 6 months, respectively) also was not significant. The response to BFI question 3 describing fatigue during the prior 24 hours also showed no statistical significance (P-value=0.94, 0.49, 0.47 at less than 1 month, 1 to 3 months, 3 to 6 months, respectively). There was no statistically significant relationship between the change in the amount of exercise and the change in level of fatigue in each follow-up period after the initial visit in multiple linear regression analysis (Table 3).
DISCUSSION
The BFI used in this research has clinical usefulness in a survey measuring severe fatigue, and was also found to be suitable for measurement of chronic, moderate, and severe fatigue in another study.18) The FSS was used with the BFI in this study because ths FSS can be used to discriminate fatigue from mental illness when used together with anxiety and depression measurement tools.17) With the BFI and the FSS, we identified the levels of overall fatigue and fatigue within one day at each visits following the initial visit, and measured the changes in each fatigue scale.
This study targeted a specific group of patients whose fatigue was not caused by an organic disease or drug. We used questionnaires to assess anxiety, depression, stress, and quality of sleep to identify mental factors related to fatigue, so the study has greater implications for effective intervention and proper treatment in many primary care outpatient clinics managing patients with fatigue.2)
We divided the follow-up periods into 3 categories after the first clinic visit, and subdivided the changes for each variable such as exercise amount and fatigue, and for multiple covariables by each time category. The possibility that fatigue could worsen with additional exercise in an early phase was also considered.
Fatigue is a subjective representation, and the definition is somewhat group-dependent;4) there are differences in the measurement of fatigue depending on the aspects of interest to the subjects being assessed. The FSS questionnaire specifically addresses whether subjects feel tired after exercise and whether they experience dysfunction after working out, but we did not use that tool efficiently, because it is limited to measurement of average final scores. The BFI is helpful in understanding the relevance of fatigue in both the general population and in patients,4) but requires further research on intervention and treatment for improvement of function in moderate or mild fatigue.18)
In general, regular exercise is traditionally known to reduce fatigue in studies on both men and women.4) In this study, the subjects were a specific group of patients who visited the fatigue clinic, but had no underlying disease. Among the subjects, 60.5% were already generally physically active or worked out with specific exercises at moderate intensity at the time of the initial visit; therefore, they could have difficulty subjectively sensing the effect of exercise intervention on their level of fatigue. If the patients already performed exercise at the beginning of the study, they rarely changed the type, frequency, and intensity of exercise in the short term. We suggested that they perform their usual exercises as much as possible, so they were unlikely to perform additional prescribed exercise.
Improper or excessive exercise can worsen symptoms in chronic fatigue patients. Follow-up for graded exercise therapy (GET) for chronic fatigue syndrome1326) is limited to the primary setting since it requires regular exercise assessment, an exercise prescription, and payments for care. Subjects in this study tended to overwork, which primarily accounted for their chronic fatigue without an underlying organic basis; therefore, we initially recommended adequate rest instead of exercise. We added GET after fatigue improved, so it was difficult to determine whether the initial visit represented the initiation of exercise therapy. This observational study had limitations. The changes in amount of exercise were measured solely according to time, and the amount of exercise was increased very slowly to prevent fatigue exacerbation. Moreover, no constructive exercise education was provided.
The response rate for this exercise-related survey was low, and the duration and frequency of exercise were often limited, especially for time-consuming and high-cost activities such as climbing and outdoor golf. Regular exercise requires mental and physical preparation and more time and space. We documented recommendations for additional physical activity in the electronic medical records, but most of these lacked responses at the subsequent visit. Seasonal change, family events, falls, and traffic accidents were also major obstacles preventing patients from performing physical activity.
Some patients were on sedatives or antidepressants at the initial visit, and others sometimes started medication during the follow-up period for symptom relief. They tended to take medication intermittently, further limiting the validity of the results of this study.
Further high quality research is needed on the effect of changes in the amount of exercise and changes in the level of fatigue; a randomized, controlled study comparing exercise therapy with no intervention, or a prospective study emphasizing GET and follow-up of an outpatient clinic-based protocol, are needed. Detailed information about questionnaire completion should be given to patients beforehand, to obtain more accurate responses in a self-reported survey, and increased use of short-form questionnaires can increase the response rate.3) The development of new tools for recording, such as smartphone applications or use of wearable devices, are also required to supplement deficiencies in survey responses.
Development of a program with a variety of exercises as interventional treatment is also important. Those working longer than 9 hours a day are known to be at higher risk of fatigue than the unemployed,2) suggesting that time is needed to recover from the fatigue caused by overwork. Regular aerobic exercise is known to be effective for the quality of sleep in healthy older people with complaints of moderate sleep disorders.27) Targeted 10-minute exercise in pilots deprived of sleep for 40 hours temporarily reduces fatigue because of increased alertness, but the fatigue eventually recurs within one hour after exercise. 28) It is important to exercise regularly in sufficient quantities in a fatigue-free state in order to obtain the benefits of exercise. This means that motivation to exercise is needed for 'too busy and have no time to exercise' people, and those who are 'always tired.' Development of a simple exercise regimen that anyone can use without time and space limitations, implementation of interval training and lifestyle changes to increase activity, and raising awareness of the negative effects of fatigue to overcome them are also needed. Environmental constraints on individual physical activity can be greater than the individual's motivation to exercise, and promotion of physical activity and exercise is needed to enable social behaviors and actions that individuals can regulate.29) Various types of exercise programs should be developed to increase motivation and compliance with physical activity; as one example, 'Wii Fit' is known to be effective in reducing fatigue in certain illnesses.30)
This study did not show a significant relationship between changes in fatigue and the amount of exercise according to the follow-up period after the initial visit to a clinic.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.