Risk Factors of Cardiovascular Disease according to Alcohol Behavioral Change after Cancer Diagnosis
Article information
Abstract
Background
Problem drinking increases the incidence of all-cause mortality and specific cancers, and persistent drinking is associated with cardiovascular disease in certain cancer survivors. This study analyzed the cardiovascular risk factors before and after diagnosis in Korean cancer survivors.
Methods
Data for the period between 2002 and 2013 were collected from the National Health Insurance Service Health-Examinee Cohort Database. Among the 27,835 patients included, those with moderate alcohol consumption before and after cancer diagnosis were excluded. Problem drinking was defined as males under 65 years consuming over 14 glasses a week, and males over 65 years or females consuming over seven glasses a week. A t-test, chi-square test, and linear regression analysis were performed for differences in cardiovascular risk factors and differences according to cancer types.
Results
There was a difference in the body mass index, systolic and diastolic blood pressure, and total cholesterol among patients who became moderate drinkers after diagnosis, but fasting blood glucose did not show any significant changes. Risk factors for cardiovascular disease were analyzed in patients with liver, stomach, rectal, and breast cancer with improved drinking behavior, and there were significant differences in body mass index, systolic and diastolic blood pressure, fasting blood glucose, and total cholesterol in stomach cancer patients.
Conclusion
Moderate drinking can lower cardiovascular risk in cancer survivors, and among the many drinking-related cancers, stomach cancer patients demonstrated significantly reduced cardiovascular risk factors.
INTRODUCTION
Alcohol consumption creates a serious medical and economic burden at the societal level and is associated with deaths from liver disease, cancer, and various chronic diseases at the individual level [1]. Together with smoking, physical activity, and diet, alcohol consumption has a large impact on health. Although the importance of moderation is emphasized, the level of alcohol consumption in South Korea is on the rise [2].
Various organizations in the United States, United Kingdom, the Netherlands, and Australia have suggested guidelines for temperance and healthy drinking [3-5]. According to the World Health Organization (WHO)’s 2014 report, alcohol consumption worldwide is on the rise. As alcohol consumption among females and young age groups, who previously consumed only small amounts, shows a steady increase, South Korea is also making various efforts to promote healthy drinking through a comprehensive citizen health promotion plan [6].
However, alcohol consumption is not all bad. When consumed in moderation, alcohol is effective in preventing mortality from cardiovascular disease (CVD), has cardioprotective effects, and is associated with a relatively reduced risk of myocardial infarction [7]. Furthermore, it has been reported to be effective in decreasing low-density lipoprotein and increasing high-density lipoprotein in regard to lipid metabolism [8]. However, this mechanism is unclear.
Alcohol consumption is also known to have a large impact on the pathogenesis and prognosis of cancer. In particular, oral, pharynx, larynx, esophageal, liver, colon, rectal, and female breast cancer are known to have causal relationships with drinking, and pancreatic and lung cancer are also expected to have associations with drinking [9]. It has been reported that in comparison with non-drinkers, the regular consumption of approximately 50 g of alcohol per day confers a three-fold increased risk of oral and pharyngeal cancers, a 1.5-fold increased risk of female breast cancer, and a 1.4-fold increased risk of colon cancer [10].
Numerous studies have analyzed alcohol consumption and the risk factors for CVD; however, few have investigated the effects drinking behavior improvement before and after a cancer diagnosis among Koreans. Hence, this study intended to investigate CVD-related risk factors among cancer survivors whose drinking behavior changed after a cancer diagnosis and the impact of cardiovascular risk factors in cancers after changes in drinking behavior.
METHODS
1. Study Population
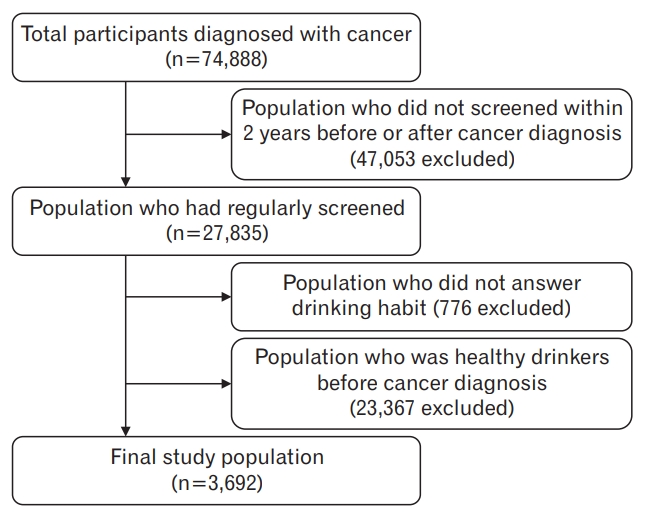
This study used the National Health Insurance Service Health-Examinee Cohort Database (2002–2013). Of the 514,866 patients (10% of the initial 5,150,000), 74,888 were diagnosed with cancer. To analyze drinking behavior changes before and after a cancer diagnosis, only examinees who had undergone health examinations within 2 years of the date of cancer diagnosis were included, and examinees whose drinking behavior survey was omitted or who drank moderately before cancer diagnosis were excluded. The final study population for the analysis of CVD-related risk factors with respect to drinking behavior changes comprised a total of 3,692 cancer survivors: 2,495 who showed behavior change to moderate drinking after a cancer diagnosis, and 1,197 who showed no drinking behavior change and continued problem drinking after a cancer diagnosis (Figure 1). Although there was no safe amount of alcohol consumption, the study aim was given to groups that should emphasize lifestyle modification when educating patients. Therefore, it was necessary to study the groups that need intervention first which is why we excluded the groups who were healthy drinkers before the cancer diagnosis. The Seoul National University Bundang Hospital Institutional Review Board (IRB) approved this study (IRB approval no., X-1701/378–902) and waived the requirement for informed consent from study participants as the NHIS database is anonymized in adherence to strict confidentiality guidelines. All experiments were performed in accordance with the relevant guidelines and regulations.
2. Variables and Measurement
1)Biological, demographic, and socioeconomic variables
The subjects’ past medical history, sociodemographic information, and life habits were collected through a self-administered questionnaire. In this study, age, sex, monthly average household income, body mass index (BMI), smoking, degree of exercise, and past medical history were analyzed as factors that could affect the pathogenesis of CVD and cancer. Examinees aged 40 years or older were grouped together within 10-year intervals. Income was divided into quartiles (Q1–Q4) according to age groups. BMI was calculated by dividing body weight by the height squared (weight [kg]/height2 [m2]) and was categorized into underweight (BMI <18.5), normal (18.5≤ BMI ≤22.9), overweight (23.0≤ BMI ≤24.9), and obese (BMI ≥25.0) according to BMI criteria for the Asian population defined by WHO. Smoking was stratified into “no” and “yes” according to the experience of smoking. As for the degree of physical activity, it was divided into “insufficient” and “sufficient,” with 3 times per week as the criterion.
2)Drinking measurement
The groups were divided on the basis of drinking amount, and the criterion for moderate drinking was an average of 14 units of alcohol or below per week for adult males and an average of seven units or below per week for adult females and males aged 65 years or older [11]. “Heavy drinking” refers to a case where the average amount per week exceeds the range of moderate drinking, and “binge drinking” refers to a case where the maximum drinking amount in one session exceeds the range of moderate drinking. Heavy and binge drinking were defined as dangerous drinking. This study used the category of problem drinking, which refers to cases where males under the age of 65 drink more than 14 units of alcohol per week, or males aged 65 years or older or females who drink more than seven units of alcohol per week [12-14].
Individuals who showed improvements in drinking behavior toward moderate drinking after their cancer diagnosis were classified into group 1 and those whose problem drinking persisted after their cancer diagnosis were classified into group 2.
3. Statistical Analysis
All analyses identified changes in risk factors according to changes in drinking behavior, and the changes were represented as real numbers, means±standard deviation, and percentages. In the comparative analysis of drinking behavior changes after cancer diagnosis and CVD-related risk factors, a Student t-test, chi-square test, and linear regression analysis were conducted. Statistical analysis was additionally adjusted for age, sex, income quantile, smoking, physical activity, and past medical history. Statistics were analyzed using Stata ver. 14.0 (Stata Corp., College Station, TX, USA), and P-values <0.05 were defined as statistically significant.
RESULTS
1. Research Subjects’ General Characteristics
In this study, 3,692 out of 74,888 patients diagnosed with cancer were included in the final analysis. The number of cases showing behavioral improvements toward moderate drinking after a cancer diagnosis (group 1) was 2,496, and the number of cases showing persistent problem drinking after a cancer diagnosis (group 2) was 1,197. In groups 1 and 2, the average age was 56.6±9.9 years and 55.0±10.2 years, respectively (P<0.001), and the proportion of males was found to be 91.50% and 95.15%, respectively (P<0.001). In addition, the proportion of those with normal BMI was 39.24% in group 1 and 33.00% in group 2 (P=0.001), but there were no intergroup differences in income quintile, physical activity, and past medical history (Table 1). Baseline characteristics for the groups excluded after cancer diagnosis were the same (Supplementary Table 1).
2. Association between Drinking Behavior Change and Cardiovascular Disease-Related Risk Factors
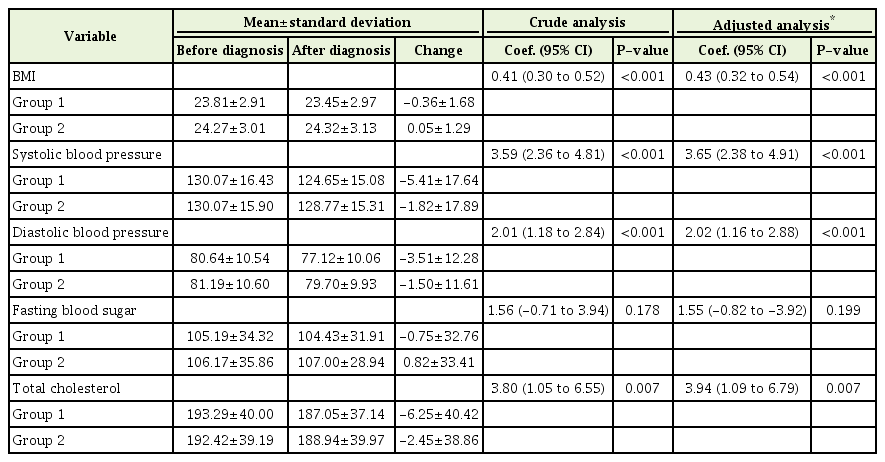
Drinking behavior changes to moderate drinking after a cancer diagnosis resulted in a tendency for the BMI to decline by -0.36±1.68 kg/m2 (P<0.001), systolic blood pressure (SBP) by -5.41±17.64 mm Hg (P<0.001), diastolic blood pressure (DBP) by -3.51±12.28 mm Hg (P<0.001), and total cholesterol (TC) by -6.25±40.42 mg/dL (P=0.007), respectively. This statistical tendency was also observed after the analysis was adjusted for age, sex, income quantile, smoking, physical activity, and past medical history (Table 2).
3. Association between Drinking Behavior Changes and Cardiovascular Disease-Related Risk Factors according to Cancer Types
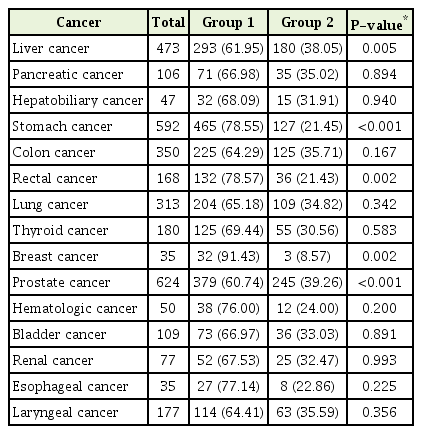
Among the cancers associated with drinking, patients with liver (P=0.005), stomach (P<0.001), rectal (P=0.002), and breast cancer (P=0.002) showed drinking behavior improvements to moderate drinking after diagnosis, while patients with head and neck, pancreatic, and colorectal cancers showed no drinking behavior improvement (Table 3). Analysis of CVD-related risk factors among patients who showed behavioral improvements to moderate drinking revealed correlations only in stomach cancer cases: with BMI (1.05; 95% CI, 0.69 to 1.41; P<0.001), SBP (9.52; 95% CI, 6.02 to 13.03; P<0.001), DBP (7.16; 95% CI, 4.78 to 9.53; P<0.001), FBS (6.45; 95% CI, 0.42 to 12.49; P=0.036), and TC (16.50; 95% CI, 7.23 to 25.77; P=0.001). In cases of liver, rectal, and breast cancers, no differences were observed in CVD-related risk factors (Table 4).
DISCUSSION
In a large survey of adult Koreans aged 40 years or older, this study investigated the changes in cardiovascular risk factors among cancer survivors according to changes in their drinking behavior through. By analyzing the cardiovascular risk factors among the existing problem drinking groups according to changes in drinking behavior after a cancer diagnosis, we found associations between drinking and cardiovascular risk factors excluding FBS (Table 2). The results do not differ from the findings of a previous study with 6,413 healthy Koreans that showed changes in SBP and DBP and an increase of FBS [15].
Although previous studies investigated cardiovascular risk factors and the causes of mortality according to specific cancer types, they were limited in terms of research on the effects of drinking behavior improvements after excessive alcohol consumption on CVD and mortality. A recent study of healthy people reported improvements in insulin resistance, blood pressure, and weight, and decreases in growth factors related to cancer after abstaining from drinking for 1 month [16]. However, not much is known about the factors that are influenced by drinking behavioral improvement among cancer survivors.
As head and neck, esophageal, stomach, pancreatic, colorectal, rectal, and breast cancers have associations with alcohol consumption, improvements in drinking behavior was associated with statistically significant differences among patients with stomach, liver, rectal, and breast cancer, along with most other cancers (Table 2). It is presumed that these effects are the result of changes in the perception brought about by a number of studies reporting the direct influence of alcohol consumption on cardiovascular and overall mortality [9,17,18].
There have been reports on cardiovascular risk factors and mortality in patients with various cancers, but reports related to alcohol consumption have been limited. In the case of patients with breast cancer, it is perceived that CVD contributes significantly to the risk of cancer death, and it is reported that death from CVD is related to cardiotoxicity and reduced estrogen [19]. Some studies, however, report that alcohol consumption has no assocaition with mortality in breast cancer survivors [20].
In cases of colorectal and rectal cancer, while some studies on the association between alcohol consumption and carcinogenesis report that there is no connection between them [21], others report that they have some connection [7] and that alcohol affects folic acid metabolism to increase the risk of colorectal cancer [12]. However, research on persistent alcohol consumption and CVD in patients with colorectal cancer has not been conducted.
Excessive alcohol consumption has been known to be a risk factor for hepatocellular carcinoma, and it is reported that its incidence has a positive correlation with the amount of drinking [9,18]. It has been reported that the mechanism for hepatocarcinogenesis caused by alcohol is related to carcinogen metabolism in the liver as well as the development of liver cirrhosis [22]. However, its association with CVD has not been clearly reported.
In cases of stomach cancer, there is no consistent evidence regarding the effects of alcohol consumption. While some studies report that the risk of stomach cancer increases as the amount of alcohol intake increases [23], others report that there is no difference [21].
The biological mechanism common to CVD and cancer caused by alcohol consumption is chronic inflammation [10]. Excessive alcohol intake increases mortality, the incidence of CVD, neutral fat, hypertension, atrial fibrillation, cardiomyopathy, and stroke, and in this process, comes to have various effects on inflammation, platelet aggregation, myocardial ischemia reperfusion, coagulation factors, vascular endothelial cell function, and anti-apoptotic pathways [17,22]. In terms of carcinogenesis, acetaldehyde, a primary product of alcohol metabolism, has toxic effects, accelerating the production of reactive oxygen and nitrogen species to impact folate and methionine metabolism and DNA repair mechanisms, causing the development of cancerous cells [16,23].
The limitations of this study are as follows. First, it is probable that the subjects’ subjective opinions were included because their lifestyle habits were collected through a self-administered questionnaire. Second, since the subjects of this study were people who underwent regular national health examinations and were interested in maintaining their health, it is possible that they showed better improvements in lifestyle habits than the ordinary populations would have. Third, while it was confirmed that changes in drinking behavior were associated with cardiovascular risk factors, whether changes in drinking behavior can affect overall mortality, and more specifically, CVD mortality, could not be elucidated. Fourth, QRISK and Framingham risk scores are used to determine an individual’s cardiovascular risk, but in performing the analysis using the National Health Insurance Service Health-Examinee Cohort Database, there were limitations in using those data.
In conclusion, it was found that excessive alcohol consumption of Korean cancer survivors increased the risk factors associated with CVD and that drinking behavior improvements after a cancer diagnosis decreased the risk factors associated with CVD. Therefore, it is necessary to conduct research on what effects these changes have on mortality.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.18.0119. Supplementary Table 1. Baseline characteristics of the exclusion population.