 |
 |
- Search
| Korean J Fam Med > Volume 43(6); 2022 > Article |
|
See commentary "Alcohol and Testosterone Deficiency in People Who Experience Facial Flushes" in Volume 43 on page 345.
Abstract
Background
This study examined the relationship between alcohol consumption and total testosterone deficiency based on facial flushing among Korean men.
Methods
A total of 314 men were included in this study and divided into non-drinkers (n=78) and drinkers (n=236). Drinkers were also divided into flushers (n=96) and non-flushers (n=140). Flushers and non-flushers were separated into two groups based on the amount of alcohol consumed: moderate drinkers (≤8 standard drinks per week) and heavy drinkers (>8 standard drinks per week). Total testosterone <3.5 ng/mL was defined as testosterone deficiency.
Results
The risk of testosterone deficiency was significantly higher in heavy drinkers who flushed than in nondrinkers (odds ratio, 4.37; 95% confidence interval, 1.20–15.88; P=0.025). However, no significant difference was observed in the risk of testosterone deficiency in non-flushers, regardless of the amount of alcohol consumed.
Testosterone deficiency can reduce the quality of life due to decreased libido, impotency, infertility, fatigue, depressed mood, decreased concentration and memory, and sleep disorders in males [1]. Among patients with chronic diseases, including diabetes mellitus, hypertension, dyslipidemia, and chronic obstructive pulmonary disease, 30% also have testosterone deficiency. Moreover, unhealthy habits, such as obesity, stress, alcohol abuse, smoking, excess caffeine intake, and a lack of exercise are associated with low levels of testosterone [2]. Among these factors, it has been demonstrated that alcohol could reduce the levels of testosterone by affecting the testis and hypothalamus-pituitary-testicular axis according to in vitro and in vivo studies [3-5]. However, in human studies, alcohol abuse has various effects on reducing the testosterone level, and these effects depend on the amount and duration of alcohol intake. Chronic excessive alcohol consumption reduces testosterone levels [4-8], but some studies claim that increased alcohol intake is independent of testosterone level [9,10]. Furthermore, others indicate that alcohol intake is positively correlated with testosterone level [11,12].
Facial flushing during alcohol intake is more often observed in East-Asians, including Koreans, than among the Western populations [13]. The East-Asian population frequently possesses a variant of the aldehyde dehydrogenase 2 (ALDH2) gene, which decomposes acetaldehyde generated from alcohol during intake [14]. Inactive ALDH2 induces the accumulation of acetaldehyde in the body, which expands facial blood vessels and causes facial flushing. This inability to metabolize alcohol increases the risk of hypertension [15], metabolic syndrome [16,17], and diabetes mellitus [18,19] in Koreans.
Most previous studies have assessed the effect of alcohol on testosterone levels based on the amount of alcohol consumed; however, few studies have considered personal differences in alcohol metabolism. Koreans have personal variations in alcohol metabolism based on genetic characteristics; therefore, it is necessary to consider both the amount of alcohol consumed and metabolic efficiency when assessing the effect of alcohol on testosterone levels. In this study, to evaluate the effect of drinking alcohol on testosterone deficiency according to an individual’s alcohol metabolism, the study participants were divided into facial flushes and non-flushes, and the relationship between alcohol consumption and testosterone deficiency was analyzed.
This study included 325 male adults whose serum total testosterone levels were measured during regular health examinations between June 2016 and December 2020 at Chungnam National University Hospital in Daejeon. Among the 325 males, some were excluded because of cancer, liver cirrhosis, chronic renal disease, thyroid diseases, and testosterone or steroid therapy. Other participants were excluded because of missing records on alcohol intake, smoking, and exercise. In total, 314 male adults were included in this study. The research protocol was approved by the institutional review board (IRB) of Chungnam Natitonal University Hospital (IRB no., 2021-07-057). Informed consent was waived as data were obtained and analyzed without patient contact.
This study was conducted using electronic medical records in a retrospective manner, and information was gathered from a questionnaire on disease history, medication history, and personal habits, such as smoking, alcohol intake, and exercise. Height and weight were measured, and body mass index (BMI) was determined by dividing the weight in kilograms by the squared height in meters.
We considered 14 g of alcohol to be a standard drink according to the criteria of the US National Institute on Alcohol Abuse and Alcoholism. One standard drink was 90 mL of 20% soju (1/4 bottle), 12 oz of beer (1 can, 355 mL), 45 mL of liquor (1 liquor drink), 150 mL of wine (one wine glass), or 300 mL of 6% makgeolli (one traditional drink). After confirming that there was no change in the amount of alcohol consumed within 1 month from the contents of the questionnaire, the amount of alcohol consumed was evaluated. Weekly alcohol intake was determined from the single alcohol intake multiplied by the number of times the participant drank per week. This weekly alcohol intake was categorized as either eight or fewer drinks (moderate drinkers) or more than eight drinks (heavy drinkers) [20]. Among alcohol drinkers, the participants were asked: “Do you experience flushing of the face immediately after drinking: always, sometimes, never, or I do not know”? If the answer was “always” or “sometimes,” never or I do not know? If the answer was “never” or “I do not know,” the participant was categorized as a non-flusher [21,22]. According to these standards, the 314 participants were divided into 78 non-drinkers, 96 flushers, and 140 non-flushers.
Smoking status was divided into current smokers (smoked within a month) and nonsmokers. Exercise was categorized into regular exercisers who performed five medium-strong exercise sessions weekly for 30 minutes or three strong exercise sessions for 20 minutes, and the rest of the participant using the standards of the US American College of Sports Medicine.
All blood tests, including total testosterone, were performed between 7–10 AM.
The general characteristics, body measurements, and blood test results of the flushers and non-flushers were compared with those of the non-drinkers. One-way analysis of variance was used to analyze continuous variables, such as age, BMI, waist circumference, blood pressure, and testosterone levels, and the Bonferroni method was used for the post-hoc test. Additionally, we applied the chi-square test for categorical variables, such as smoking history and exercise habits. To identify the alcohol intake and flushing factors that affected total testosterone levels, we performed a multiple regression analysis with age, waist circumference, hypertension, diabetes mellitus, dyslipidemia, weekly alcohol intake, smoking history, and exercise habits as covariates. We separated the participants into five groups as follows: 78 non-drinkers, 71 flushers with ≤8 weekly standard drinks, 79 non-flushers with ≤8 weekly standard drinks, 25 flushers with >8 weekly standard drinks, and 61 non-flushers with >8 weekly standard drinks. We then used the chi-square and Bonferroni post-hoc tests to compare the prevalence of testosterone deficiency among the five groups. We defined testosterone deficiency as levels <3.5 ng/mL. We analyzed the risk of testosterone deficiency in the drinking groups (flushers and non-flushers) compared with the non-drinking group using logistic regression analysis. Model 1 did not compensate for confounding variables, whereas model 2 compensated for age, waist circumference, hypertension, diabetes, dyslipidemia, smoking, and exercise habits. Statistical significance was set at P-value <0.05, and all data were analyzed using IBM SPSS ver. 28.0 software (IBM Corp., Armonk, NY, USA).
Among the 314 participants, there were 78 non-drinkers and 236 drinkers. Of the 236 drinkers, there were 96 flushers and 140 non-flushers. The average ages of the non-flushers (55.2±8.6 years, P<0.001) and flushers (56.4±10.3 years, P<0.05) were significantly lower than the average age of the non-drinkers (60.6±8.7 years). The total testosterone level was not significantly different across the groups of non-drinkers (5.1±1.7 ng/mL), flushers (4.9±1.5 ng/mL), and non-flushers (4.9±1.8 ng/mL). The amount of drinking was 6.5±9.7 and 12.2±13.5 drinks per week among flushers and non-flushers, respectively, and there was a significant difference (P<0.001). No significant differences in hypertension, diabetes mellitus, dyslipidemia, smoking, or exercise habits were observed between the groups (Table 1).
Table 2 presents the multivariate linear regression with age, waist circumference, hypertension, diabetes mellitus, dyslipidemia, weekly alcohol intake, smoking, and exercise among the non-drinkers, drinkers, flushers, and non-flushers. Age was correlated with total testosterone levels among non-drinkers (β=0.243, P=0.029), drinkers (β=0.158, P=0.019), and flushers (β=0.209, P=0.048). Waist circumference was negatively correlated with total testosterone level, regardless of flushing observations (drinkers: β=-0.250, P<0.001; flushers: β=-0.276, P=0.008; non-flushers: β=-0.230, P=0.009). No significant difference in waist circumference was observed among the non-drinkers (β=-0.295, P=0.378). Weekly alcohol intake was negatively correlated with total testosterone levels among flushers (β=-0.283, P=0.004). Hypertension was positively correlated among non-drinkers (β=0.096, P=0.001), but negatively correlated among drinkers (β=-0.137, P=0.045) and flushers (β=-0.182, P=0.049). Dyslipidemia was negatively correlated among non-drinkers (β=-0.160, P=0.011), drinkers (β=-0.187, P=0.003), and non-flushers (β=-0.209, P=0.012).
The average total testosterone level was analyzed in the groups after weekly alcohol intake was separated by eight weekly standard drinks. Figure 1A shows that the flusher group that consumed >8 drinks had significantly lower total testosterone level of 4.0 ng/mL compared to the non-drinkers (5.1 ng/mL, P=0.040) and flushers who consumed ≤8 drinks (5.2 ng/mL, P=0.023). Additionally, there was a significant difference when five groups with total testosterone <3.5 ng/mL were analyzed using chi-square test (P=0.026), and the differences between the five groups were analyzed using the Bonferroni post-hoc test. The testosterone deficiency in flushers that consumed >8 weekly (36.0%) and those that consumed ≤8 weekly drinks (8.5%) had significantly different proportions of testosterone deficiency compared to others (15.4%, 21.5%, and 18.0% in non-drinkers, non-flushers with ≤8 weekly drinks and non-flushers with >8 weekly drinks, respectively) at P-value <0.05 level (Figure 1B). In contrast, if there was no flush, no difference in the proportion of participants with testosterone deficiency was detected in non-flushers, regardless of the alcohol intake.
The odds ratios (ORs) of testosterone deficiency in the flusher group when weekly alcohol consumption was ≤8 drinks were 0.51 in model 1 (95% confidence interval [CI], 0.18–1.43; P=0.201); and 0.62 in model 2 (95% CI, 0.19–2.03; P=0.428). When weekly alcohol consumption was >8 drinks, the ORs became 3.10 in model 1 (95% CI, 1.11–8.60; P=0.030) and 4.37 in model 2 (95% CI, 1.20–15.88; P=0.025) (Table 3). When weekly alcohol consumption was ≤8 drinks, the non-flusher group had ORs of 1.51 in model 1 (95% CI, 0.67–3.41; P=0.322) and 1.85 in model 2 (95% CI, 0.71–4.80; P=0.206). When weekly alcohol consumption was >8 drinks, the ORs of the non-flusher group were 1.21 in model 1 (95% CI, 0.49–2.97; P=0.677) and 1.81 in model 2 (95% CI, 0.57–5.74; P=0.317) (Table 3).
This study investigated the effects of alcohol intake on testosterone deficiency based on facial flushing. However, there are contrasting reports on the relationship between alcohol intake and testosterone levels. In general, chronic excessive alcohol intake is known to decrease testosterone levels; however, moderate alcohol intake results in various effects on testosterone levels. Most previous studies only considered the amount of alcohol consumed, and did not consider individual alcohol metabolism. According to our study, there was a weak negative correlation between the total testosterone levels and alcohol consumption in flushers. The drinker groups that flushed and consumed >8 standard drinks per week (112 g of alcohol per week) had a 4.37 times higher risk of testosterone deficiency than the non-drinker group.
Testosterone is a hormone synthesized by the Leydig cells in the testis according to feedback from the hypothalamus-pituitary-testicular axis. The testosterone level is known to be reduced by alcohol, which affects the testis and central level [3]. Ethanol and its metabolic by-product, acetaldehyde, reduce the synthesis of testosterone directly in the Leydig cells or indirectly by generating active oxygen radicals. Moreover, ethanol and acetaldehyde limit the production and secretion of gonadotropins from the hypothalamus, decreasing testosterone levels [5-7,21]. Facial flushing is activated by the acetaldehyde that accumulates within the body, which is enabled by inactive ALDH2. Therefore, if facial flushing develops during drinking, the individual will endure the harmful effects of ethanol and acetaldehyde for a longer duration [22,23]. For this reason, it is possible that the average level of testosterone was low and the risk of testosterone deficiency increased only in the flusher group at the relatively low eight standard drinks (112 g of alcohol) per week in our study compared to other studies. Some studies have reported that Koreans who develop a drinking flush have a higher risk of hypertension [15], metabolic syndrome [16,17], diabetes mellitus [18,19], high rheumatoid factors [24], and high intraocular pressure [25] even if they do not drink much.
We did not detect a significant difference between the total testosterone levels of drinkers and nondrinkers (Table 1). Svartberg et al. [9] and Watts et al. [10] reported no relationship between alcohol intake and total testosterone levels, which aligns with our results. However, our results revealed a weak negative correlation between total testosterone level and alcohol consumption in flushers and an increased risk of testosterone deficiency among heavy drinkers (eight drinks per week) who flushed. This result also agrees with various reports claiming that consuming alcohol reduces testosterone level [4-8]. Van Thiel et al. [5] reported that a healthy adult male who consumed a pint of whiskey (473 mL) in a single day developed low total testosterone levels after 72 hours, and the level dropped to that of alcoholics. Muthusami and Chinnaswamy [7] observed that alcoholics (who consumed 180 mL/d of whiskey 5 days per week for more than 1 year) had lower total testosterone levels than non-drinkers. Most studies that have analyzed the inverse relationship between alcohol intake and testosterone levels have used cases of chronic alcoholism or acute excessive consumption of alcohol. Therefore, these studies showed that alcohol intake affects testosterone levels only during biologically risky alcohol consumption, which is not typical of alcohol consumption. In our study, total testosterone levels and the prevalence of testosterone deficiency were higher in the heavy drinking group (>8 drinks per week) with flushing, but the amount of alcohol consumed (17.7 drinks or 248 g per week) was lower than that in previous studies. This is thought to be due to the lower alcohol metabolism of Korean men than that of Westerners, as described above. In flushers with moderate drinking (≤8 drinks per week), testosterone deficiency was lower than that in other groups, but total testosterone levels were not significantly different from those in the other groups. Therefore, this is considered the result of an insufficient number of study participants, rather than the positive effect of moderate drinking.
This study has some limitations that should be discussed. First, this study was a retrospective analysis; therefore, causality between alcohol consumption and testosterone levels could not be established. Second, this study enrolled a lower proportion of heavy drinkers, which could have underestimated or overestimated the effects of alcohol on testosterone levels. Third, the participants were limited to a population that had undergone health examinations; therefore, the participants did not represent all Korean male adults.
Despite these limitations, this study is significant because it considered personal alcohol metabolism, as observed by facial flushing, to analyze the effect of alcohol consumption on total testosterone levels. In conclusion, heavy drinkers who flushed had an increased risk of testosterone deficiency after consuming >8 standard drinks per week (112 g/wk). Therefore, we recommend that drinkers who flush should limit their alcohol intake to eight or fewer standard drinks per week or not drink.
Figure. 1.
Total testosterone level and prevalence of testosterone deficiency according to alcohol consumption in flushers and non-flushers. (A) One drink was equivalent to 14 g alcohol. Values are presented as the mean±standard deviation. *P=0.040 compared to non-drinkers; †P=0.023 compared to flushers who had eight or fewer drinks per week by analysis of variance with the Bonferroni post-hoc test. (B) Testosterone deficiency was defined as <3.5 ng/mL. Values are presented as numbers (%). Five groups were analyzed using the chi-square test (P=0.026), and the differences among the five groups were analyzed using the Bonferroni post-hoc test. ‡These values were significantly different compared to the others (P=0.05) in the Bonferroni post-hoc test.
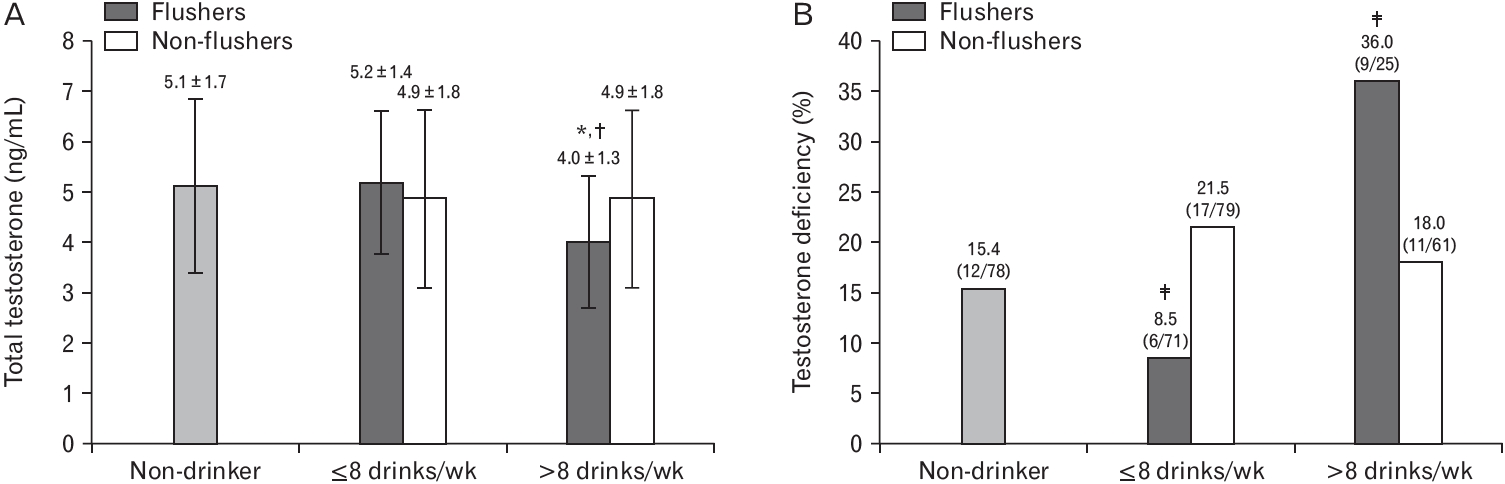
Table 1.
General subjects characteristics
| Characteristic | Non-drinkers (n=78) |
Drinkers (n=236) |
|
|---|---|---|---|
| Flushers (n=96) | Non-flushers (n=140) | ||
| Age (y) | 60.6±8.7 | 56.4±10.3** | 55.2±8.6*** |
| Body mass index (kg/m2) | 24.0±2.8 | 24.4±2.7 | 24.5±3.2 |
| Waist circumference (cm) | 85.9±9.1 | 87.4±7.9 | 86.1±8.4 |
| Body fat (%) | 23.6±6.0 | 24.1±5.4 | 22.9±5.4 |
| Systolic blood pressure (mm Hg) | 124.1±15.4 | 123.5±13.7 | 124.6±13.1 |
| Diastolic blood pressure (mm Hg) | 75.8±10.6 | 76.8±10.5 | 76.8±9.7 |
| Triglyceride (mg/dL) | 131.3±77.0 | 128.7±66.3 | 155.0±114.1 |
| Total cholesterol (mg/dL) | 182.2±34.8 | 200.1±36.1** | 196.6±36.5* |
| Low-density lipoprotein cholesterol (mg/dL) | 112.1±29.7 | 125.8±31.8* | 118.3±33.1 |
| High-density lipoprotein cholesterol (mg/dL) | 46.8±11.2 | 52.2±13.3* | 52.9±14.3** |
| Glucose (mg/dL) | 103.3±18.0 | 102.4±20.0 | 106.6±20.9 |
| Hemoglobin A1c (%) | 5.7±0.7 | 5.7±1.0 | 5.7±0.9 |
| Aspartate aminotransferase (IU/L) | 25.2±7.7 | 27.1±10.3 | 30.7±12.5**,† |
| Alanine aminotransferase (IU/L) | 25.4±13.1 | 27.1±15.5 | 33.1±18.8**,† |
| Gamma-glutamyltransferase (IU/L) | 30.4±20.8 | 41.4±37.4 | 67.6±91.8***,†† |
| Total testosterone (ng/mL) | 5.1±1.7 | 4.9±1.5 | 4.9±1.8 |
| Hypertension | |||
| No | 44 (56.4) | 61 (63.5) | 82 (58.6) |
| Yes | 34 (43.6) | 35 (36.5) | 58 (41.4) |
| Diabetes mellitus | |||
| No | 63 (80.8) | 84 (87.5) | 121 (86.4) |
| Yes | 15 (19.2) | 12 (12.5) | 19 (13.6) |
| Dyslipidemia | |||
| No | 40 (51.3) | 54 (56.3) | 71 (50.7) |
| Yes | 38 (48.7) | 42 (43.8) | 69 (49.3) |
| Smoking | |||
| No | 21 (65.6) | 26 (50.0) | 32 (59.3) |
| Yes | 11 (34.4) | 26 (50.0) | 22 (40.7) |
| Exercise | |||
| Irregular | 22 (68.8) | 43 (82.7) | 38 (70.4) |
| Regular | 10 (31.2) | 9 (17.3) | 16 (29.6) |
| Drinking amount (drinks per week) | - | 6.5±9.7*** | 12.2±13.5***,††† |
Table 2.
Associations between total testosterone level and related factors according to alcohol consumption and facial flushing
| Variable |
Total testosterone |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Non-drinkers (n=78) |
Drinkers (n=236) |
Flushers (n=96) |
Non-flushers (n=140) |
|||||
| β | P-value | β | P-value* | β | P-value* | β | P-value* | |
| Age (y) | 0.243 | 0.029 | 0.158 | 0.019 | 0.209 | 0.048 | 0.118 | 0.182 |
| Waist circumference (cm) | -0.295 | 0.378 | -0.250 | <0.001 | -0.276 | 0.008 | -0.230 | 0.009 |
| Hypertension | 0.096 | 0.001 | -0.137 | 0.045 | -0.062 | 0.545 | -0.182 | 0.049 |
| Diabetes mellitus | -0.401 | 0.140 | 0.038 | 0.558 | 0.150 | 0.129 | -0.034 | 0.693 |
| Dyslipidemia | -0.160 | 0.011 | -0.187 | 0.003 | -0.164 | 0.099 | -0.209 | 0.012 |
| Current smoker | 0.063 | 0.554 | 0.099 | 0.145 | 0.072 | 0.492 | 0.080 | 0.378 |
| Regular exercise | -0.137 | 0.209 | 0.015 | 0.801 | -0.034 | 0.716 | 0.017 | 0.835 |
| Drinking amount (drinks/wk) | - | - | -0.071 | 0.273 | -0.283 | 0.004 | 0.035 | 0.691 |
| R2 | 0.316 | 0.185 | 0.258 | 0.206 | ||||
Table 3.
The odds ratios of testosterone deficiency according to alcohol consumption in flushers and non-flushers
REFERENCES
1. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35.


2. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1715-44.



3. Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. Substance abuse and male hypogonadism. J Clin Med 2019;8:732.



4. Anderson RA, Willis BR, Oswald C, Reddy JM, Beyler SA, Zaneveld LJ. Hormonal imbalance and alterations in testicular morphology induced by chronic ingestion of ethanol. Biochem Pharmacol 1980;29:1409-19.


5. Van Thiel DH, Gavaler JS, Cobb CF, Santucci L, Graham TO. Ethanol, a Leydig cell toxin: evidence obtained in vivo and in vitro. Pharmacol Biochem Behav 1983;18 Suppl 1:317-23.

6. Ida Y, Tsujimaru S, Nakamaura K, Shirao I, Mukasa H, Egami H, et al. Effects of acute and repeated alcohol ingestion on hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal functioning in normal males. Drug Alcohol Depend 1992;31:57-64.


7. Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril 2005;84:919-24.


8. Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 2006;50:291-6.

9. Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol 2003;149:145-52.


10. Watts EL, Appleby PN, Albanes D, Black A, Chan JM, Chen C, et al. Circulating sex hormones in relation to anthropometric, sociodemographic and behavioural factors in an international dataset of 12,300 men. PLoS One 2017;12:e0187741.



11. Jensen TK, Swan S, Jorgensen N, Toppari J, Redmon B, Punab M, et al. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum Reprod 2014;29:1801-9.



12. Hansen ML, Thulstrup AM, Bonde JP, Olsen J, Hakonsen LB, RamlauHansen CH. Does last week’s alcohol intake affect semen quality or reproductive hormones?: a cross-sectional study among healthy young Danish men. Reprod Toxicol 2012;34:457-62.


14. Agarwal DP, Harada S, Goedde HW. Racial differences in biological sensitivity to ethanol: the role of alcohol dehydrogenase and aldehyde dehydrogenase isozymes. Alcohol Clin Exp Res 1981;5:12-6.


15. Jung JG, Kim JS, Kim YS, Oh MK, Yoon SJ. Hypertension associated with alcohol consumption based on the facial flushing reaction to drinking. Alcohol Clin Exp Res 2014;38:1020-5.



16. Jung JG, Kim JS, Yoon SJ, Oh MK. Relationships among alcohol consumption, facial flushing response, and metabolic syndrome in healthy men. Ann Epidemiol 2012;22:480-6.


17. Seo YR, Kim JS, Kim SS, Jung JG, Yoon SJ. Association between alcohol consumption and metabolic syndrome determined by facial flushing in Korean women. Korean J Fam Med 2021;42:24-30.



18. Jung JG, Kim JS, Oh MK. The role of the flushing response in the relationship between alcohol consumption and insulin resistance. Alcohol Clin Exp Res 2010;34:1699-704.


19. Kim J, Kim JS, Kim SS, Jung JG, Yoon SJ, Seo YR, et al. Influence of facial flushing on pre- or type 2 diabetes risk according to alcohol consumption in Korean male. Korean J Fam Med 2020;41:153-60.




20. Lee S, Kim JS, Jung JG, Oh MK, Chung TH, Kim J. Korean alcohol guidelines for moderate drinking based on facial flushing. Korean J Fam Med 2019;40:204-11.




21. Jana K, Jana N, De DK, Guha SK. Ethanol induces mouse spermatogenic cell apoptosis in vivo through over-expression of Fas/Fas-L, p53, and caspase-3 along with cytochrome c translocation and glutathione depletion. Mol Reprod Dev 2010;77:820-33.


22. Yokoyama A, Muramatsu T, Ohmori T, Kumagai Y, Higuchi S, Ishii H. Reliability of a flushing questionnaire and the ethanol patch test in screening for inactive aldehyde dehydrogenase-2 and alcohol-related cancer risk. Cancer Epidemiol Biomarkers Prev 1997;6:1105-7.

23. Shin CM, Kim N, Cho SI, Sung J, Lee HJ. Validation of alcohol flushing questionnaires in determining inactive aldehyde dehydrogenase-2 and its clinical implication in alcohol-related diseases. Alcohol Clin Exp Res 2018;42:387-96.










