INTRODUCTION
Analgesic nephropathy (AN) is a form of chronic renal insufficiency caused by long-term regular ingestion of one or more analgesic medications. Although a well-recognized condition to most physicians, its etiopathogenesis remains controversial and the particular agents known to induce AN and the cumulative doses required have not been established. Formerly one of the commoner causes of end-stage renal failure (ESRF), there has been a marked decline in the prevalence and incidence of AN in recent decades.1,2) At its peak in the 1980s, AN accounted for 15% to 20% of cases of ESRF, but has fallen to approximately 1% in Western countries today.3,4,5,6,7)
An association between non-opiate analgesic drugs and chronic renal impairment was first hypothesized in German literature in 19538) based on observations that analgesic abusers frequently developed chronic kidney disease (CKD). Phenacetin was singled out as the culprit in early reports and its widespread withdrawal from the market is a possible explanation for the dramatic decline of AN. Legislation installed globally throughout the late twentieth century restricting prescription of combination analgesics may also have contributed to this reduced incidence, and the nephrotoxicity of various non-phenacetin preparations continues to be speculated.9,10,11)
The true prevalence of AN is difficult to estimate because definitive diagnoses are rarely made. There are no validated objective diagnostic criteria. In the absence of other causes, it is often a diagnosis of exclusion in patients with renal impairment who report a history of analgesic abuse. Most patients are asymptomatic and middle age or older. They frequently describe a history of chronic pain, typically headaches or low back pain.12) Patients usually admit to having consumed regular analgesic medicines for many months or years, although they may deny or underreport their usage. Abnormal renal function is usually a serendipitous finding. Radiologic signs described in AN include atrophic kidneys, renal papillary calcification, and irregular renal contours. This combination of features is seldom seen in other diseases. No histologic changes are diagnostic of AN, but papillary necrosis and chronic interstitial nephritis are both characteristic of the condition.4,5,10)
Despite a relatively large quantity of observational evidence, the relative contribution of specific drugs in the development of AN remains undetermined. Randomized controlled trials are lacking and research so far has relied upon experimental animal models or epidemiological human studies with conflicting outcomes. This narrative review intends to summarize the available literature.
PHENACETIN
Phenacetin is an analgesic antipyretic compound that is metabolized to paracetamol and other reactive intermediates. Formerly in widespread distribution, phenacetin was gradually withdrawn from markets worldwide because of adverse effects, notably its asserted propensity to induce renal impairment. Phenacetin products were the most widely sold non-prescription analgesics in the 1960s and 1970s.12) Staged withdrawal took place throughout the world roughly between 1960 and 1980. Historical epidemiologic data identifying close correlation between AN prevalence and phenacetin sales forms a large part of the argument connecting the two. Numerous observational publications, primarily from Australia and Europe, have demonstrated a sharp decrease in AN incidence coinciding with the withdrawal of phenacetin mixtures.1,3,10,11,13,14,15,16) It was also shown in Australia that the prevalence of AN was highest in regions of heaviest phenacetin consumption. 17)
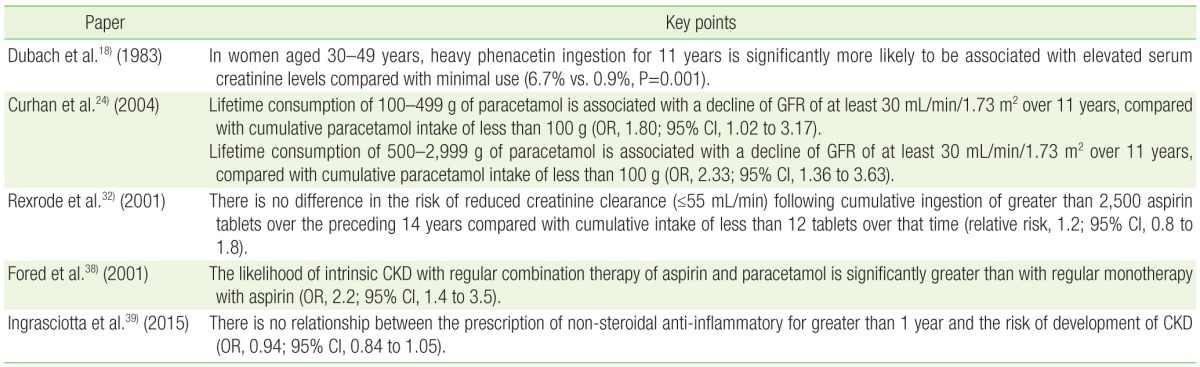
Daily phenacetin users are more likely to have renal impairment than controls. The largest trial investigating this relationship is a cross sectional analysis conducted on 7,311 young women in Switzerland.3) At 11 years follow-up, heavy phenacetin users were considerably more likely to develop increased serum creatinine levels than were the controls who did not consume phenacetin (6.7% vs. 0.9%, P<0.001).18) Although causation was not ascertained, this result certainly confirms phenacetin abuse as a risk factor for poor renal function. It is reasonable to suspect that patients developing CKD suffered greater comorbidities than did healthy controls and were therefore more likely than the controls to require phenacetin, rather than the inverse relationship. Equivalently, phenacetin users had higher mortality from cancer and cardiovascular disease in this study. Presumably their general ill health produced a need for analgesia, and one would hesitate to attribute a causative role to phenacetin ab initio.
Phenacetin was closely linked to nephropathy in a 1982 Australian case control study.19) In this blinded study, nephrology outpatients with radiographic features of AN were far more likely to have consumed large quantities of analgesic mixtures in a lifetime, almost all containing phenacetin, than controls with unsuspicious imaging (relative risk, 17; 95% confidence interval [CI], 8.5 to 34.7). A strength of this study was the average duration of analgesic use of greater than 19 years, which increases the likelihood that the exposure preceded the disease. Furthermore, the mean cumulative consumption was 24 kg, markedly higher than any other study of phenacetin nephrotoxicity. However, the investigators did not determine if any patients in the AN study group had underlying renal pathology, and structural CKD cannot be definitively attributed to analgesic abuse without exclusion of such patients.
Phenacetin compounds remain in contemporary use in some developing areas. Recent Chinese data suggests that heavy phenacetin users in their population are more vulnerable to chronic renal impairment compared with controls.20) A cross-sectional survey of more than 47,000 residents identified that twice-weekly use of analgesia, of which phenacetin substrates comprised 76.9% of responses, for greater than two years at any time previously was associated with a significantly higher risk of subnormal renal function (odds ratio [OR], 2.36; 95% CI, 1.28 to 4.37). However, the study results require cautious interpretation. Subgroup analysis of phenacetin and non-phenacetin users was not performed. Furthermore, after adjusting for confounders including cardiovascular disease and diabetes, there was no statistically significant relationship between analgesia and CKD, reported as low glomerular filtration rate (GFR) or albuminuria (OR, 1.21; 95% CI, 0.87 to 1.67 and OR, 1.13; 95% CI, 0.92 to 1.40, respectively).
Concerns of nephrotoxicity originate from studies involving phenacetin in combination preparations. The risk of CKD with phenacetin alone has never been reported in the literature.3) Indeed, phenacetin has never been released as a single agent and instead has been prescribed exclusively in combination products. Experimental rat studies of long-term phenacetin monotherapy at therapeutic doses have not demonstrated nephrotoxicity, although chronic supratherapeutic administration has repeatedly been found to produce papillary necrosis. 21,22,23) Whether experimental animal findings equate to the context of clinical practice in humans is uncertain.
PARACETAMOL
Paracetamol is one of the most widely consumed drugs in modern medicine. It is an antipyretic analgesic metabolite of phenacetin. Anecdotal and observational research has highlighted a potential role of paracetamol in the development of AN. It is clear from numerous studies that regular paracetamol use is associated with a greater risk of renal impairment. In one prospective study, lifetime cumulative intake of more than 500 g of paracetamol conferred a higher risk of a decline in GFR of at least 30 mL/min/1.73 m2 over 11 years (OR, 2.23; 95% CI, 1.36 to 3.63).24) In a multicenter retrospective study, daily paracetamol ingestion was associated with higher risk of CKD compared with non-consumers (OR, 3.2; 95% CI, 1.05 to 9.80).25) Certainly the findings of these papers underscore that excessive paracetamol ingestion and CKD frequently occur together, although that does not assign the principle of causation.
Dialysis patients are more likely than control groups to have consumed long-term regular paracetamol. In an investigation by Perneger et al.,26) individuals reporting lifetime consumption of 1,000ŌĆō4,999 paracetamol tablets were more likely to be current dialysis users than those who had taken 999 tablets or less (OR, 2.0; 95% CI, 1.3 to 3.2). The likelihood of dialysis was even higher in subjects reporting lifetime ingestion exceeding 5,000 tablets (OR, 2.4; 95% CI, 1.2 to 4.8). Patients listed on the Michigan Kidney Registry, a state-wide dialysis register, are also more likely to report chronic paracetamol abuse than random civilians (OR, 2.66; 95% CI, 1.04 to 6.82).27) As in other epidemiologic studies, these reports are vulnerable to reverse causality; ESRF patients are more likely to require paracetamol as a consequence of their general condition than are the healthy control population, and paracetamol use per se may be unrelated to the evolution of kidney impairment. Patients with known renal diagnoses were not excluded in these papers, which is important given the relative rarity of AN as a cause for ESRF.
Several publications have not demonstrated an increased risk of CKD despite paracetamol abuse.28,29) The National Health and Nutritional Examination Survey (NHANES), a large cross-sectional analysis of more than 8,000 random community residents, found no statistically significant difference in the chance of poor renal function between abusers of paracetamol and non-users.2,29)
ASPIRIN
Research has failed to identify any consistent relationship between the protracted use of aspirin alone and the development of AN. No correlation was discovered in any of the aforementioned controlled studies by Curhan et al.,24) Perneger et al.26) or Sandler et al.,25) nor was habitual aspirin use accompanied by GFR decline in the NHANES. Several small longitudinal studies of heavy aspirin users in rheumatology outpatient cohorts have also not documented any increased risk of renal impairment.30,31) The largest cohort trial investigating aspirin is the case control study by Rexrode et al.32) Researchers recruited 11,032 healthy young men to complete a survey of analgesic use annually for 14 years. Total aspirin intake was calculated for the study period and serum creatinine measured in the first and final years. Patients consuming combination drugs were excluded and a multivariate analysis was performed to account for medical comorbidities. No connection between serum creatinine levels and aspirin consumption was detected, including in the highest-use group who consumed in excess of 2,500 doses (OR, 0.83; 95% CI, 0.50 to 1.39). This study was well conducted. Its strengths included longitudinal design, relatively large cohort size, the good health of the participants compared with populations in other studies, and its exclusion of patients with alternative explanations for their renal impairment.
Okada et al.33) published a cohort study in 2016 that failed to connect long-term aspirin monotherapy with renal insufficiency. In a cohort of diabetic patients, daily use of low-dose aspirin for a median of 8.5 years was not associated with lower GFR (annual change ŌłÆ0.8 mL/min/1.73 m2 vs. ŌłÆ0.9 mL/min/1.73 m2, P=0.2) nor a higher rate of dipstick-positive albuminuria (hazard ratio, 1.17; 95% CI, 0.96 to 1.38), compared with no aspirin use. The Okada study followed a complicated protocol, in which randomized arms receiving aspirin or no aspirin for 4.4 years in a separate trial were followed at that study's conclusion for a further 4.1 years. During the non-randomized phase, participants could commence or cease aspirin at their treating doctor's discretion. At 8.5 years, 18% of the aspirin group was not consuming aspirin, while 11% of the no-aspirin group was taking daily aspirin. The null hypothesis advertised by this study is therefore less than absolute, but is nonetheless helpful as observational evidence.
A positive association between aspirin abuse and CKD has been revealed in at least one study on humans. A European case control study published in 2005 surveyed 583 consecutive dialysis patients on aspirin usage.34) Compared with controls matched for age and gender and not receiving dialysis, the OR for being in the dialysis group after reporting chronic aspirin ingestion was significant at 1.56 (95% CI, 1.05 to 2.30). That finding is diluted because inclusion into the intervention group required only 15 consecutive days of aspirin use, and nearly twice as many aspirin consumers in the dialysis group were taking aspirin for cardiovascular disease than in the control group (12% vs. 7%, respectively).
Mixtures containing both paracetamol and aspirin are associated with greater renal toxicity than either agent used alone.35,36) This was confirmed in a Belgian prospective controlled study of 400 primary care patients followed for seven years between 1984ŌĆō1992.37) Participant inclusion necessitated a minimum lifetime consumption of at least 1,000 doses of aspirin, paracetamol, or phenacetin, alone or in combination. Renal function impairment developed in 6% of patients consuming analgesia compared with 1% of non-users (RR, 6.1; 95% CI, 1.4 to 25.9). Almost all mixtures contained paracetamol and less than 9% contained phenacetin, and no agent in monotherapy was associated with a decline in kidney function. A Swedish population study carried out between 1996ŌĆō1998 reported similar findings.38) It compared 926 subjects with persistently raised serum creatinine levels, automatically identified by computerized pathology laboratories, with 998 controls. Consumers of paracetamol-aspirin combinations were more than twice as likely to have raised creatinine levels compared to levels in consumers of aspirin monotherapy (OR, 2.2; 95% CI, 1.4 to 3.5).
NON-STEROIDAL ANTI-INFLAMMATORIES
The majority of large epidemiologic studies have failed to substantiate an association between heavy non-steroidal anti-inflammatory (NSAID) use and CKD in healthy individuals. The prospective paper by Curhan et al.24) found no increased risk for renal impairment at 11 years follow-up despite regular NSAID use. Regular ibuprofen users in the NHANES had no change in renal function compared with controls. 29) There was also no link demonstrated in the case control study by Ibanez et al.34) nor the large 14 year prospective study by Rexrode et al.32) In a recent Italian case control study of approximately 2,000 patients with CKD and nearly 8,000 matched controls, no association was identified between high intake of NSAIDs and renal insufficiency (OR, 0.94; 95% CI, 0.84 to 1.05).39) A multicenter European report of 4,529 dialysis patients and controls did not find abuse of any common non-phenacetin analgesics to increase the risk of ESRF (OR, 1.02; 95% CI, 0.81 to 1.28).40) A post hoc review of subjects from the high consumption group of that study, defined as lifetime ingestion of greater than 3 kg of non-opioid non-phenacetin analgesic agents, also failed to show any correlation between NSAID monotherapy and the development of ESRF.41)
Sandler et al.42) did observe a moderately increased risk of unexplained CKD among regular NSAID consumers versus controls in their multicenter study (OR, 2.1; 95% CI, 1.1 to 4.1). In this investigation, researchers compared the reported past analgesic usage of a cohort of 554 patients with CKD with that of a control group of 516 random community members. It is unusual that the relationship was not dose-dependent, and after subgroup analysis the only cohort to maintain statistical significance was men older than 65 years of age. A large shortcoming with this paper was the lack of adjustment for patients simultaneously consuming other narcotic and non-narcotic analgesic agents. It should also be noted that the CKD group were hospitalized patients with high degrees of comorbidity and ostensibly high analgesia requirements. Additionally, 95% of NSAID users consumed various NSAIDs rather than a select drug, making incrimination of a particular agent impossible. The few who consumed a single agent used ibuprofen or indomethacin: no matched controls were found for the ibuprofen group and the indomethacin group had no increased risk of CKD.
In the large registry study by Perneger et al.,26) the prominent finding was a potential association between paracetamol and ESRF. The NSAID group overall had no statistically significant connection to dialysis rates, but multivariate analysis revealed that the subgroup with lifetime consumption of greater than 5,000 NSAID tablets had an OR for ESRF requiring dialysis of 8.8 (95% CI, 1.1 to 71.8). The CI of this result is unreliably wide, the high-use group was small at 18 subjects, and there was no adjustment for comorbidities. Furthermore, the study populations were quite different: the intervention group contained mostly black males compared with predominantly white females in the control arm.
Chronic repeated intake of NSAIDs may hasten disease progression in patients with pre-existing kidney insufficiency. In a prospective population study of 10,184 patients with known CKD, regular NSAID use was associated with a moderately increased risk of a GFR decline greater than 15 mL/min/1.73 m2 over 2.75 years follow-up (OR, 1.26;95% CI, 1.04 to 1.53).43) Several smaller cohort and case control studies have supported these findings.44)
DISCUSSION
The development of renal insufficiency as a consequence of chronic analgesic consumption remains unproven despite a large body of epidemiologic research. The hypothesis of AN is untested in randomized trials and supportive data is largely circumstantial (Table 1). Non-narcotic drugs comprise a broad class and the role of specific agents in the genesis of AN is unknown. Furthermore, the precise duration and cumulative dosage necessary to induce nephrotoxicity has not been defined.
The available evidence relatively strongly implicates phenacetin substances in the development of AN. The aggregate of epidemiologic and experimental observations form a persuasive argument for causation. Many authors ascribe the declining incidence of AN over recent decades to the withdrawal of phenacetin preparations, which were last available in the United States in 1983.45) As a single agent, phenacetin has never been shown to induce renal injury; however, it has never been available as a single product. In any case, the clinical relevance of phenacetin continues to decline in contemporary practice since its removal from the market.
The weight of evidence implies a link between paracetamol abuse and the development of AN; however, observed trends are guarded and rather imprecise. Multiple controlled studies have confirmed that frequent users are at higher risk of renal impairment in the long term. Combination mixtures containing paracetamol and aspirin are more highly associated with nephrotoxicity than either agent consumed alone. Aspirin theoretically potentiates the renal effects of paracetamol by altering renal blood flow and drug metabolism.
The reported nephrotoxicity of long-term NSAIDs or aspirin is unsubstantiated by the present literature. Aspirin monotherapy at therapeutic doses has not been associated with an increased risk of renal disease in most observational studies, some of which are large and soundly executed. Papers reporting an increased risk are weakened by inclusion of patients prescribed aspirin for cardiovascular indications. Such patients are clearly at greater risk of kidney disease. While NSAIDs are a well-recognized cause of acute kidney injury, there are no robust data linking long-term use with CKD. The majority of commentaries refute such a relationship and at most the renal toxicity of chronic NSAID use would be very small.
Although there is a considerable body of research discussing the pathophysiology of AN, causality is difficult to establish for any agent because study design in this field is heterogeneous, with inherent limitations and biases. Selection bias is a fundamental weakness of observational evidence that leads to retro-causation. In the current context, the possibility remains that patients with CKD or those predisposed to developing CKD have a greater demand for analgesia than controls. In effect, analgesia itself may bear no influence on kidney health. It is well-recognized that heavy consumers of analgesics have higher levels of chronic illness than a standard population.46) Furthermore, because renal insufficiency is typically insidious and analgesic use is widespread, any link based on epidemiologic data is likely to be coincidental rather than causal. Minimal research has shown that analgesic exposure antedated the onset of kidney disease, an absolute requirement for establishing causation. Finally, the endpoint in many papers on the subject is ESRF rather than AN or some lesser degree of renal impairment. This provides data insufficient to support or refute the hypothesis since AN progresses to ESRF relatively infrequently.
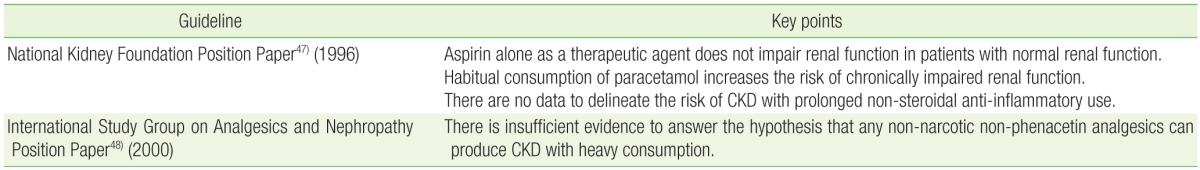
Several major authorities have attempted to publish guidelines outlining best practice in light of available evidence (Table 2). A position statement released by the National Kidney Foundation in 1996 concluded that data provided from case control studies is sufficient to confirm a weak association between long-term paracetamol ingestion and AN.47) It also concluded that based on the strengths and limitations of the data, aspirin alone at therapeutic dosages does not appear to induce CKD. No comment was made on the importance of NSAIDs in the pathobiology of AN because of inadequate evidence. It was contended that long-term therapy with aspirin or NSAIDs worsens prognosis in patients with pre-existing renal impairment. The subject was revisited in 1999 by a committee of international experts that convened in Germany.48) They concluded that the theory of nephropathy caused by any non-phenacetin non-opioid analgesic cannot be confidently supported or refuted. Phenacetin admixtures were the only agents believed capable of generating nephropathy beyond reasonable doubt. However, several important papers have been published since these meetings, which should also be taken into consideration.24,29,32,34)
Most nephrologists accept AN as a real entity despite an absence of firm evidence.3) There is a pressing need for randomized trials to assess the true incidence of renal impairment related to specific agents, although the ethics and practicalities may make such a proposal unfeasible. While much of the accumulated supporting data is inconclusive, clinical practice should be founded on the best available evidence. Most authorities suggest that prevention is a rational approach to management, and until outstanding issues are settled it may be a matter of good clinical judgment to consider all non-opioid analgesics nephrotoxic and to avoid prolonged use where possible.
CONCLUSION
Analgesic nephropathy is defined as chronic renal insufficiency caused by long-term heavy exposure to one or more analgesic medications. This contentious entity has been the object of clinical and experimental research for decades and its relationship with particular analgesic agents remains unproven. Phenacetin was strongly associated with renal function impairment; however, its clinical importance is declining in contemporary practice. The level of evidence involving other agents is poor. Protracted usage of paracetamol and combination mixtures containing paracetamol and aspirin probably produce AN; however, there is no substantive evidence that prolonged consumption of aspirin or NSAIDs as single agents results in renal impairment. Until randomized studies can conclusively establish the renal safety of analgesic drugs, it may be best clinical practice to consider all agents to be nephrotoxic with chronic use and to discourage such consumption where possible.