|
 |
- Search
| Korean J Fam Med > Volume 43(5); 2022 > Article |
|
Abstract
Background
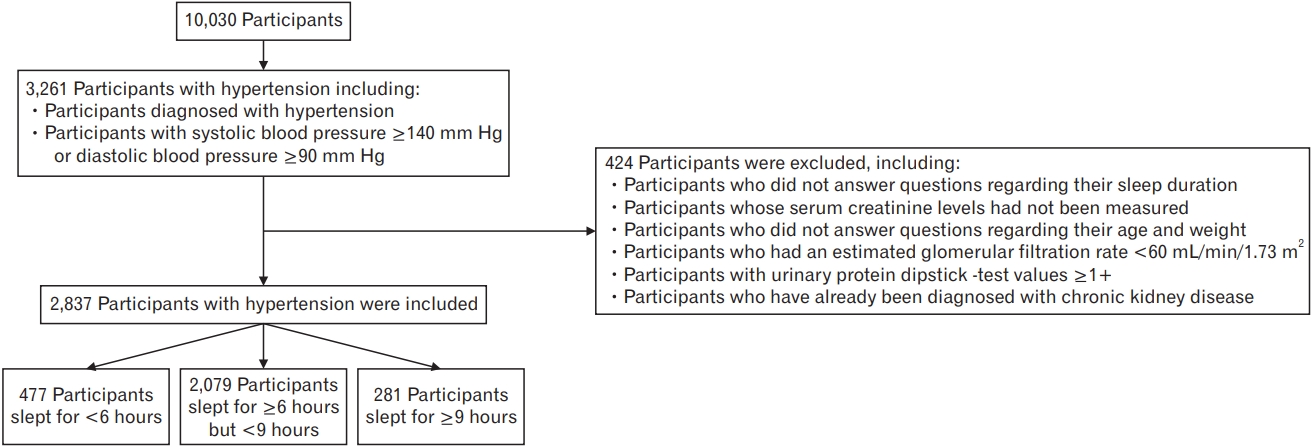
Methods
Results
Table 1.
Table 2.
Model 1: age, sex, baseline estimated glomerular filtration rate, baseline systolic blood pressure, and baseline diastolic blood pressure had been adjusted for. Model 2: covariates adjusted for in model 1 and diabetes status, dyslipidemia status, cancer-history status, body mass index, smoking status, alcohol-consumption status, physicalactivity duration, snoring status, and Epworth Sleepiness Scale score had been for in model 2.
HR, hazard ratio; CI, confidence interval.
Table 3.
| Variable |
Sleep duration (h) |
||||
|---|---|---|---|---|---|
|
<6 |
≥6 but <9 |
≥9 |
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Baseline blood pressure | |||||
| Well-controlled† (n=176) | 1.02 (0.28–3.69) | 0.972 | Reference | 2.45 (0.52–11.53) | 0.257 |
| Poorly controlled hypertension‡ (n=990) | 1.56 (1.02–2.36) | 0.038 | Reference | 0.84 (0.41–1.72) | 0.638 |
| Diabetes | |||||
| No (n=1,047) | 1.39 (0.91–2.10) | 0.124 | Reference | 0.91 (0.50–1.67) | 0.762 |
| Yes (n=119) | 0.27 (0.07–1.03) | 0.056 | Reference | 0.21 (0.01-3.08) | 0.255 |
| Age (y) | |||||
| <65 (n=982) | 1.36 (0.88–2.09) | 0.163 | Reference | 0.75 (0.34–1.65) | 0.474 |
| ≥65 (n=184) | 1.54 (0.72–3.30) | 0.267 | Reference | 0.94 (0.36–2.43) | 0.900 |
| Body mass index (kg/m2) | |||||
| <25 (n=569) | 1.60 (0.85–3.02) | 0.146 | Reference | 0.42 (0.16–1.10) | 0.077 |
| ≥25 (n=597) | 1.51 (0.92–2.48) | 0.107 | Reference | 1.38 (0.63–3.05) | 0.422 |
* Age, sex, baseline estimated glomerular filtration rate, baseline systolic blood pressure, baseline diastolic blood pressure, diabetes status, dyslipidemia status, cancer-history status, body mass index, smoking status, alcohol-consumption status, physical-activity duration, snoring status, and Epworth Sleepiness Scale scores had been adjusted for in this analysis (model 2).
Table 4.
| Variable |
Sleep duration (h) |
||||
|---|---|---|---|---|---|
|
<6 |
≥6 but <9 |
≥9 |
|||
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Baseline blood pressure | |||||
| Well-controlled† (n=240) | 1.29 (0.67–2.50) | 0.450 | Reference | 0.59 (0.24–1.46) | 0.257 |
| Poorly controlled hypertension‡ (n=901) | 0.89 (0.64–1.25) | 0.512 | Reference | 1.05 (0.75–1.47) | 0.781 |
| Diabetes | |||||
| No (n=1,044) | 0.90 (0.66–1.22) | 0.485 | Reference | 0.90 (0.65–1.26) | 0.548 |
| Yes (n=97) | 1.99 (0.74–5.36) | 0.172 | Reference | 1.22 (0.29–5.20) | 0.788 |
| Age (y) | |||||
| <65 (n=887) | 0.81 (0.56–1.16) | 0.251 | Reference | 1.02 (0.68–1.51) | 0.940 |
| ≥65 (n=254) | 1.24 (0.78–1.98) | 0.366 | Reference | 0.87 (0.52–1.46) | 0.599 |
| Body mass index (kg/m2) | |||||
| <25 (n=437) | 0.75 (0.46–1.23) | 0.262 | Reference | 1.21 (0.71–2.06) | 0.481 |
| ≥25 (n=704) | 1.02 (0.71–1.46) | 0.927 | Reference | 0.77 (0.51–1.15) | 0.203 |
* Age, sex, baseline estimated glomerular filtration rate, baseline systolic blood pressure, baseline diastolic blood pressure, diabetes status, dyslipidemia status, cancer-history status, body mass index, smoking status, alcohol-consumption status, physical-activity duration, snoring status, and Epworth Sleepiness Scale scores had been adjusted for in this analysis (model 2).
REFERENCES
- TOOLS