Relationship between Serum Testosterone Level and Carotid Intima-Media Thickness among Korean Men and Postmenopausal Women
Article information
Abstract
Background
Given that the role of serum testosterone on incident cardiovascular disease has been uncertain, it is necessary to find out the relationship between serum testosterone and carotid atherosclerosis.
Methods
The study participants included 1,302 Korean adults (873 men and 429 postmenopausal women) who participated in the Healthy Twin Study and were not receiving androgen deprivation therapy. The participants were classified into three groups: men aged <40 and ≥40 years and postmenopausal women. Total testosterone (TT) and sex hormone-binding globulin (SHBG) concentrations were measured using electrochemiluminescence immunoassays, and free testosterone (cFT) levels were calculated using Vermeulen’s method. Carotid intima-media thickness (IMT) was measured at three levels using a high-resolution B-mode ultrasound equipped with a 7-MHz linear transducer. The associations between sex hormone concentrations and carotid IMT were evaluated using a mixed linear regression analysis.
Results
After adjusting for cardiovascular risk factors, TT was found to be inversely associated with common carotid IMT in men aged ≥40 years, with a 4.5% decrease in common carotid IMT for every one-standard deviation increase in TT concentration (P=0.0063). In contrast, TT was not significantly associated with carotid IMT in all segments in men aged <40 years and postmenopausal women. Additionally, SHBG and cFT were not associated with carotid IMT in any segment.
Conclusion
The significant association between TT level and common carotid IMT in men aged ≥40 years suggests that decreased testosterone levels are involved in the development of atherosclerosis in men.
INTRODUCTION
Endogenous testosterone levels in men affect sexual function, physical force, behavior, and cognitive function [1]. Serum testosterone levels in men gradually decrease with age [2], a reduction that may be associated with many age-associated health problems, including dyslipidemia, vascular endothelial dysfunction, abdominal obesity, and metabolic syndrome [3,4]. Low serum testosterone levels have been associated with a greater risk of cardiovascular disease (CVD) and subclinical atherosclerosis in men [5-7]. However, the relationship between serum testosterone and CVD is unclear in postmenopausal women, as both higher [8,9] and lower [10,11] testosterone levels have been associated with a greater CVD risk.
Carotid intima-media thickness (IMT) has been shown to be associated with cardiovascular risk factors such as total cholesterol, smoking, and high blood pressure (BP) [12,13], suggesting that carotid IMT is an indicator of subclinical atherosclerosis [12]. To date, several studies have evaluated the association between serum testosterone levels and CVD risk as determined by carotid IMT. For example, a French multicenter cohort study showed that low serum testosterone levels were associated with increased carotid IMT in elderly men with high (≥2 mg/L) serum C-reactive protein (CRP) concentrations but not in elderly men with lower serum CRP levels [14]. Additionally, an inverse relationship between serum testosterone levels and carotid IMT was observed in middle-aged Japanese men [15]. However, other studies have failed to show an association between testosterone levels and carotid IMT [4,16]. Thus, it remains unclear whether a reduction in serum testosterone level is associated with subclinical atherosclerosis. Additionally, to date, few studies have evaluated this relationship in Asian women who may have different CVD risk factors than the more frequently studied populations, including Asian men and Western men and women.
Considering that the role of serum testosterone on incident CVD risk has been uncertain, it is necessary to find the association between serum testosterone and carotid IMT among healthy populations, which may give quantitative evidence of the role of testosterone in the development of subclinical atherosclerosis.
Therefore, the present study investigated the association between serum total testosterone (TT) concentration and carotid IMT in each segment of Korean men and postmenopausal women.
METHODS
1. Study Participants
This cross-sectional study was conducted among Korean men and postmenopausal women who underwent measurements of carotid IMT and serum TT and sex hormone-binding globulin (SHBG) concentrations, while participating in the Healthy Twin Study between 2009–2014. Details of the study design and protocol of the Healthy Twin Study have been published. 17) Briefly, the Healthy Twin Study is a multicenter cohort study of adult (≥30 years) same-sex twins and their first-degree adult family members recruited from the general population.
Men who were diagnosed with prostate cancer or had received treatment for prostate cancer were excluded. Furthermore, both men and women were excluded if they had been previously diagnosed with myocardial infarction. Postmenopausal status of the women was based on self-reports. Women who reported no menstrual cycles within the previous 12 months were considered postmenopausal if they had previously experienced natural menopause, received hormone replacement therapy, had undergone bilateral oophorectomy, or were aged ≥55 years. Of the women who met these criteria for postmenopausal status, those aged <40 years and those with a serum estradiol level >50 pg/mL were excluded. A schematic flow diagram of the cross-sectional study is shown in Figure 1.
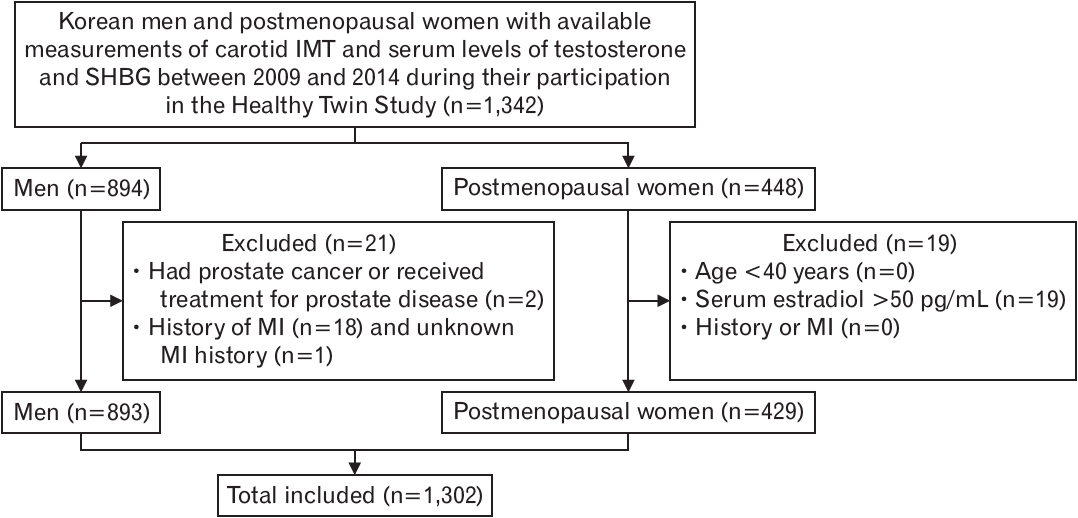
Schematic flow diagram. IMT, intima-media thickness; SHBG, sex hormonebinding globulin; MI, myocardial infarction.
Written informed consent was obtained from all the participants. The study protocol was approved by the Institutional Review Board of Samsung Medical Center (2011-10-025).
2. Carotid Intima-Media Thickness Measurement
Carotid IMT was measured during the end-diastolic phase between the P and Q waves on electrocardiogram traces using an automated IMT package with a high-resolution B-mode Vivid (General Electric, Horten, Norway) or EKO 7 (Medison, Cypress, CA, USA) carotid ultrasound machine equipped with a 7-MHz linear transducer. Carotid IMT was scanned on the far walls of three segments, located 10–20 mm proximal to the tip of the flow divider into the common carotid artery (CCA), at the carotid bifurcation (BIF), beginning at the tip of the flow divider and extending 10 mm proximal to the flow divider tip, and at the proximal 10 mm of the internal carotid artery (ICA). The combined carotid IMT in each participant was calculated as the mean of both sides of the three carotid artery segments.
Reliability of the IMT measurements was assessed in 14 randomly selected individuals by repeatedly measuring the IMT with two carotid ultrasound machines (Vivid and EKO 7). The intraclass correlation coefficients for CCA, BIF, and ICA were 0.93, 0.86, and 0.90, respectively.
3. Hormone Assays
Venous blood samples were collected between 8−10 AM after an overnight fast. The sera were separated immediately by centrifugation and frozen at -70°C. TT and SHBG concentrations were measured immediately after thawing the frozen serum in a central laboratory. The TT concentrations were determined by chemiluminescence immunoassay (ADVIA Centaur XP kits; Siemens, Erlange, Germany), and the SHBG concentrations were determined by electrochemiluminescence immunoassay (MODULAR ANALYTICS E170 analyzer; Roche, Basel, Switzerland). The minimum measurable concentrations at the central laboratory were 0.35 nmol/L for both TT and SHBG. The inter-assay coefficients of variation were <7.6% for TT and <2% for SHBG. Free testosterone (cFT) concentrations were calculated from the measured levels of TT and SHBG using the Vermeulen’s method [18].
4. Measurement of Covariates
Body weight and height were measured by trained research assistants using standard protocols, with the average of repeated measurements used to calculate the body mass index (BMI, weight divided by height squared [kg/m2 ]). BP was measured twice for each participant by a trained research assistant using a mercury sphygmomanometer, and the averages of these measurements were used for the analysis.
Serum concentrations of glucose, hemoglobin A1C, total cholesterol, and high-density lipoprotein cholesterol (HDL-C) were measured using commercial kits at a designated central laboratory. Non-HDL-C concentration was calculated by subtracting HDL-C from the total cholesterol concentration. Information on prostate cancer diagnosis, medications being taken, including those for hypertension and diabetes mellitus, hormone deprivation treatment, and health behaviors, including smoking habits, alcohol consumption, and physical exercise were collected using a self-administered questionnaire. Hypertension was defined as BP ≥140/90 mm Hg or taking antihypertensive medication, and diabetes was defined as a fasting serum glucose concentration ≥126 mg/dL, hemoglobin A1C concentration ≥6.5%, or use of a glucose-lowering agent. Smoking habits were classified into three groups: never smokers, ex-smokers, and current smokers. Alcohol consumption was categorized into two groups: current drinkers and nondrinkers. Physical exercise, defined as moderate or high intensity regular exercise activity, was categorized into three groups according to frequency per week (<1, 1–2, or ≥3).
5. Statistical Analysis
The measured carotid IMTs were log-transformed to approximate a normal distribution. The age-adjusted concentration of each sex hormone was calculated using a regression model to exclude the effects of age on these associations. The linearity of the distribution of carotid IMT in the three segments and covariates was assessed separately in men and women according to TT concentration quartiles using Spearman’s correlation analysis and the Cochran–Mantel–Haenzel test.
Multivariable adjusted associations of carotid IMT with TT and cFT were evaluated using mixed linear regression analyses, in which random effects (household and sibling effects) and fixed effects (alcohol consumption, smoking status, physical exercise, BMI, hypertension, diabetes mellitus, and non-HDL cholesterol) were considered. The percent differences in carotid IMT per unit increase in standard deviation (SD) of TT and cFT concentrations were calculated by subtracting one from the exponentiated β coefficients, which were assessed using the mixed linear model for log-transformed carotid IMT measurements.
The mean age of male participants in this study was 46 years. Accordingly, we divided the men into two groups of 40 years of age. The Northern Manhattan Stroke Study also used the age division criteria of 40 years as an appropriate age to determine the association with cardiovascular risk factors [19]. The association between testosterone level and carotid IMT risk was repeatedly evaluated in three groups of participants: men aged <40 years, men aged ≥40 years, and postmenopausal women. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA), with two-sided P-values of <0.05 defined as statistically significant.
RESULTS
This study included 1,302 participants, comprising 873 men (401 men aged <40 years and 472 men aged ≥40 years) and 429 postmenopausal women. Table 1 presents the general characteristics of the study participants. Mean age of male and female participants were 46.0±14.7 years and 61.3±8.2 years, respectively. A total of 21.3% of men and 32.4% of women had comorbid hypertension, and 7% of men and 9.3% of women had diabetes mellitus. Mean±SD level of serum TT and cFT concentrations were 593.4±215.5 nmol/L and 10.93±4.2 nmol/L in men and 37.5±24.8 nmol/L and 0.45±0.4 nmol/L in women, respectively.
Table 2 shows the relationship between carotid IMT and covariates according to the TT quartiles in men. Age showed an inverse relationship with TT level. The mean carotid IMTs of all three segments, non-HDL cholesterol concentrations, and BMI decreased linearly as the TT levels increased. The percentage of men with diabetes mellitus tended to decrease as the TT concentration increased.
Table 3 shows the relationship between carotid IMT and covariates according to the TT quartiles in women. Similar to the findings in men, age and TT levels were inversely related in women. However, the TT concentration was not significantly associated with carotid IMT in any of the segments.

Distribution of measured variables according to total testosterone level in postmenopausal women (N=429)
Table 4 shows the multivariable-adjusted associations between TT and carotid IMT in men aged <40 years, ≥40 years, and postmenopausal women. A significant inverse association was observed between TT and common carotid IMT in men aged ≥40 years (P=0.0063), with a 1-SD increase in TT accompanied by a 4.5% decrease in IMT of the CCA. However, this association between carotid IMT and TT was not found in the other segments of the carotid artery, and cFT was not associated with carotid IMT in any of the three segments. Moreover, TT and cFT were not associated with carotid IMT in any of the three segments in men aged <40 years and postmenopausal women.
DISCUSSION
This study among Korean adults showed that TT concentration was significantly inversely associated with common carotid IMT in men aged ≥40 years, and this association was independent of the major CVD risk factors. Although this association was not observed in other carotid segments or in younger men and postmenopausal women, the finding in men aged ≥40 years suggests that testosterone may have a beneficial role in protecting older men from developing atherosclerosis.
Other studies have also reported an inverse association between testosterone levels and carotid IMT, suggesting that increased testosterone concentration may reduce CVD risk. For example, a population-based cross-sectional study of 1,482 men aged 25–84 years showed that TT but not cFT concentration was inversely associated with age-adjusted combined IMT of CCA and BIF, after adjusting for smoking, physical activity, BP, and lipid level [20]. However, this association disappeared after further adjustment for BMI. A multivariable analysis of 239 Finnish men aged 40–70 years revealed that IMT at the CCA was inversely correlated with TT level after adjusting for age, BMI, lipid level, and BP [21]. Additionally, low serum testosterone levels have been associated with increased cardiovascular mortality in men [7].
However, other studies have failed to show significant associations between testosterone and carotid IMT or CVD. For example, a cohort study of 2,290 Norwegian men with a mean age of 66 years found no association between testosterone and CVD development [16]. Although that study observed an inverse cross-sectional association between TT and carotid plaque area independent of major confounders, TT and plaque area progression were not associated during the 7-year follow-up period. Additionally, both cross-sectional and prospective analyses found no association between TT levels and carotid IMT. The Atherosclerosis Risk in Communities Study found that testosterone was not associated with carotid IMT after adjusting for covariates, although testosterone was significantly associated with cardiovascular risk factors [4]. Taken together, these findings indicate that the association between sex hormones and carotid IMT remains unclear.
Interestingly, we found that testosterone levels were associated with carotid IMT in men aged ≥40 years. This association was not observed in younger men, possibly because carotid arteriosclerosis is unlikely to occur in this population. Additionally, it may be difficult to observe this association in very old men with advanced atherosclerotic changes because carotid IMT is a preclinical surrogate marker of atherosclerosis [12,13]. Therefore, it may be appropriate to investigate the association between testosterone levels and carotid IMT in middle-aged men.
Carotid IMT is associated with several cardiovascular risk factors, including total cholesterol concentration, smoking, and high BP [12,13]. Thus, the association between low testosterone levels and individual CV risk factors, such as hypertension [22], diabetes [23], and metabolic syndrome [24] may explain the association between testosterone and carotid IMT. However, studies, including the present study, found an independent association between testosterone and IMT, even after adjusting for these cardiovascular risk factors.
Several mechanisms have been suggested to underlie the association between testosterone and atherosclerosis. For example, serum testosterone concentration is inversely correlated with the concentration of vascular cell adhesion molecule-1 (VCAM-1), which plays an important role in the development of atherosclerosis [25]. The inverse association between serum testosterone levels and VCAM-1 suggests that the progression of atherosclerosis may be suppressed by the anti-inflammatory effect of testosterone [15]. Furthermore, testosterone has been found to block lipid uptake, reduce lipoprotein‐lipase activity, and decrease the conversion of preadipocytes to adipocytes, resulting in visceral fat accumulation [26]. Low testosterone levels may also affect glucose transport and reduce antioxidant activity [3].
The present study has several strengths. First, it was a community-based study that included healthy men without a history of androgen depravation therapy, allowing an examination of the association between physiological concentrations of testosterone and carotid IMT. Second, this study evaluated FT and TT separately. Third, this study included a wide range of cardiovascular risk factors as covariates.
The present study has several limitations. First, owing to its cross-sectional observational design, it was difficult to determine the cause-and-effect relationships of the associations observed in this study. Second, hormone concentrations were measured only once and by radioimmunoassay rather than by mass spectrometry, which is considered to be more accurate. Third, we used the calculated cFT level with Vermeulen’s method instead of directly measuring serum cFT. The majority of TT is bound to SHBG, and a small proportion of TT is a physiologically active free form, which was presented as cFT in the present study. However, it has been suggested that it is questionable whether cFT can actually represent bioavailable testosterone [27,28]. Additionally, the relationship between cFT and metabolic syndrome seems to be weaker than that between cFT and TT [29]. Therefore, the finding of our study that TT but not cFT has a significant inverse association with carotid IMT may support the true role of T in atherosclerosis. Fourth, carotid IMT was measured by several sonographers using two types of machines, which may have resulted in a measurement bias. However, because the intraclass correlations for assessing interobserver and intermachine repeatability were moderate to high, significant bias was less likely to be involved. Finally, participants who took medications for hair loss could not be excluded from our study because of lack of relevant data.
In conclusion, this Korean population study found a significant inverse association between TT concentration and common carotid IMT in men aged ≥40 years independent of CV risk factors. In contrast, TT concentration was not associated with carotid IMT in men aged <40 years or postmenopausal women. These findings suggest that testosterone may play a role in the development of CVD in middle-aged men.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.



