 |
 |
- Search
| Korean J Fam Med > Volume 44(1); 2023 > Article |
|
Abstract
Background
Human immunodeficiency virus (HIV), the agent responsible for acquired immunodeficiency syndrome, remains a worldwide public health problem. Therefore, this study aimed to assess Portuguese healthcare studentsŌĆÖ knowledge of HIV, identify risk behaviors for HIV transmission, and assess the frequency of HIV testing and its dissemination by general practitioners.
Methods
A cross-sectional observational study was conducted using an anonymous questionnaire published online. The questionnaire was administered to Portuguese healthcare students who voluntarily agreed to participate in the study after clarifying its objectives and procedures.
Results
Most students were aware of the three main HIV transmission routes; however, 66.3% were unaware of the level A recommendation for HIV screening in adolescents and adults. In addition, 59.6% of the students were never informed by their general practitioner about HIV screening tests or risk behaviors for HIV transmission. Of the sample, 78.9% had never been tested for HIV infection. Of the 74.6% of sexually active students, 82.0% had or had already had unprotected sex (without a condom). Men showed riskier sexual behaviors than women. None of the inquiries reported intravenous drug use, and most students (69.9%) never shared personal objects.
Human immunodeficiency virus (HIV), the causative agent responsible for acquired immunodeficiency syndrome (AIDS), remains a public health problem worldwide. By 2020, statistics revealed that worldwide and across all ages, there were 37.7 million people diagnosed with HIV, 1.5 million new HIV infections, and 680,000 deaths due to AIDS [1].
The natural history of HIV infection is a reflection of the interaction between the hostŌĆÖs immune system and viral replication. It is characterized by a gradual decrease in the TCD4+ lymphocyte count, with a progressive depletion of the host immune system cells [2]. A confirmed case can be classified into one of five stages (0, 1, 2, 3, or unknown). Stage 0 is an acute viral infection, and stages 1, 2, and 3 are defined according to the TCD4+ lymphocyte count (Ōēź500, 200ŌĆō449, and <200/mm3, respectively). If the patient presents with an AIDS-defining illness, stage 3 is initially assigned, regardless of the TCD4+ lymphocyte count [3].
The United States Preventive Services Task Force recommends that laboratory screening for HIV infection should be performed in all adolescents and adults (recommendation level A) [4]. Similarly, according to the Portuguese guidelines, laboratory screening for HIV infection should be performed in all individuals aged 18 to 64 years (grade of recommendation I) [5]. The main goals of optimizing screening test prescription in clinical practice are early diagnosis and treatment, implementing measures to prevent HIV transmission to others, decreasing the number of HIV-infected newborns, and decreasing the stigma associated with testing [5].
The current standard treatment is antiretroviral therapy (ART), which consists of a daily combination of three oral drugs: two nucleoside analog reverse transcriptase inhibitors and a third drug that can be an integrase inhibitor, a protease inhibitor, or non-nucleoside reverse transcriptase inhibitor [6].
ART, when administered in the acute phase of the disease, prolongs the patientŌĆÖs life expectancy and reduces the risk of HIV transmission to others [6]. Despite the great advances in medicine that ART has brought, there is currently no cure for HIV infection or vaccine to prevent it [7].
The three main routes of HIV transmission are sexual, parenteral, and vertical. Sexual transmission prevails as the predominant route [8-10].
HIV is transmitted through unprotected sex (without a condom) with HIV-infected individuals. Although all sexual practices carry an infection risk, unprotected anal sex carries a higher risk than unprotected vaginal sex. Multiple sexual partners and short intervals between them, co-infection with other sexually transmitted infections (STI), and oral sex involving exposure to virus-infected semen, are also associated with an increased risk of infection [9,10].
Intravenous drug use is another risk factor for HIV transmission owing to the sharing of contaminated needles or other equipment used to prepare these drugs. Parenteral transmission is also associated with sharing utensils that have been in contact with the virus, including personal items. There is still a risk of HIV transmission through blood transfusions or organ transplants in some countries [9,10]; however, in Portugal, this is practically nonexistent [11].
HIV transmission is mainly associated with individual behaviors; however, it is not exclusive. Healthcare workers are at constant risk of HIV transmission when providing care to HIV-infected patients. Therefore, prevention depends crucially on public awareness campaigns and the minimization of risk behaviors [12].
Therefore, this study aimed to assess Portuguese healthcare studentsŌĆÖ knowledge about HIV infection, identify possible risk behaviors of this population that predispose them to HIV transmission, and assess the frequency of conducting and disseminating the HIV screening test by their general practitioners (GP). This study also aims to raise awareness of this issue by promoting the self-reflection of each person so that they become aware of their behaviors and attempt to change them, demystifying the prejudices related to sexual health and HIV testing.
This cross-sectional observational study was conducted based on a questionnaire released online. We emailed all the healthcare universities in Portugal requesting authorization and dissemination of the survey to students; however, many universities were not cooperative. Thus, the dissemination of the surveys was essentially carried out with the help of the studentsŌĆÖ associations of the universities and through direct contacts who disseminated it through social media groups of Portuguese healthcare students. We collected the studentsŌĆÖ answers between March and August 2021.
This study was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra (Comiss├Żo de ├ētica da ARS do Centro; CE-044/2021).
There are 55,000 healthcare students in Portugal. A significant sample for a 95% confidence interval and 5% margin of error would be 382 participants. The questionnaire was administered anonymously, with all confidentiality measures regarding data collection and analysis undertaken, and no personal data that could identify the person were recorded. After obtaining informed consent, the participants voluntarily completed the questionnaire. Data were stored in a database/researcher file for subsequent analyses.
The study was aimed at healthcare students attending the first and second cycles, undergraduate, and integrated masterŌĆÖs degrees in Portugal, who agreed to participate voluntarily in the study after being informed about its objectives and procedures. We chose this sample because it is a young population that are future healthcare professionals, thus representing a group at higher risk of HIV transmission and who will play an important role in raising awareness about this matter.
For data collection, the participants completed a questionnaire. The questionnaire included identifying data such as age, sex, field of study, college, academic year in which the participant was at the time of the filling of the form, and previously attended courses. Personal data regarding sexual practices and orientation were also collected.
Subsequently, there was a section on HIV screening, in which two subsections were on knowledge and risk behaviors for sexual and parenteral HIV transmission. Regarding vertical HIV transmission, only the studentsŌĆÖ knowledge was assessed.
Statistical analysis was performed using the IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA). The significance level ╬▒ was set at 0.05; thus, for P<0.05, a statistically significant association was considered to exist between variables. The normality of the distribution of the continuous variables was analyzed using the Kolmogorov-Smirnov test. Continuous variables were represented by their means and standard deviations, and categorical variables by their relative (%) and absolute (number) frequencies. The chi-square (Žć2) and FisherŌĆÖs exact tests, and the Mann-Whitney and Kruskal-Wallis tests were used to determine statistically significant differences between categorical and continuous variables, respectively, among students of different sexes, fields of study, and sexual orientations.
The sample consisted of 611 Portuguese health students: 79.5% were women, and 20.5% were men. The ages ranged from 18 to 43 years, with a mean age of 21.2┬▒3.0 years. The participants were distributed between their first and sixth years of college, and 20.8% of the students had attended another course. The median number of years of schooling was 3.0┬▒1.6. The distribution of the participants s by the area of study is shown in Table 1.
Thirty-five percent of the students were studying in Coimbra, 20.9% in Lisbon, 9.7% in Oporto, 6.5% in Covilh├Ż, 4.7% in Bragan├¦a, 4.3% in Vila Nova de Famalic├Żo, 4.1% in Leiria, 3.9% in Set├║bal, 2.8% in Viana do Castelo, 2.6% in Braga, 2.3% in the Azores, 2.1% in Vila Real, and 0.7% in Aveiro.
Regarding sexual orientation, 88.5% of the participants were heterosexual, 4.6% bisexual, 4.1% homosexual, and the remaining 2.8% were included in ŌĆ£other/I do not know/I do not want to answer.ŌĆØ
Students were questioned about HIV testing and the dissemination of information about risk behavior prevention by the GP. The results are described in Table 2, which compare medical students with those from other healthcare fields. HIV testing was also compared between participants of different sexual orientations. The results are presented in Table 3.
StudentsŌĆÖ knowledge regarding the main routes of HIV transmission, risk behaviors that may predispose them to infection, and HIV screening test recommendations are described in Table 4. The results were compared between medical students and those from other healthcare fields.
We analyzed studentsŌĆÖ risk behaviors that predetermined an increased risk of contracting or transmitting HIV sexually. No significant differences were found between medical students and those from other healthcare fields. Differences between men and women are shown in Table 5.
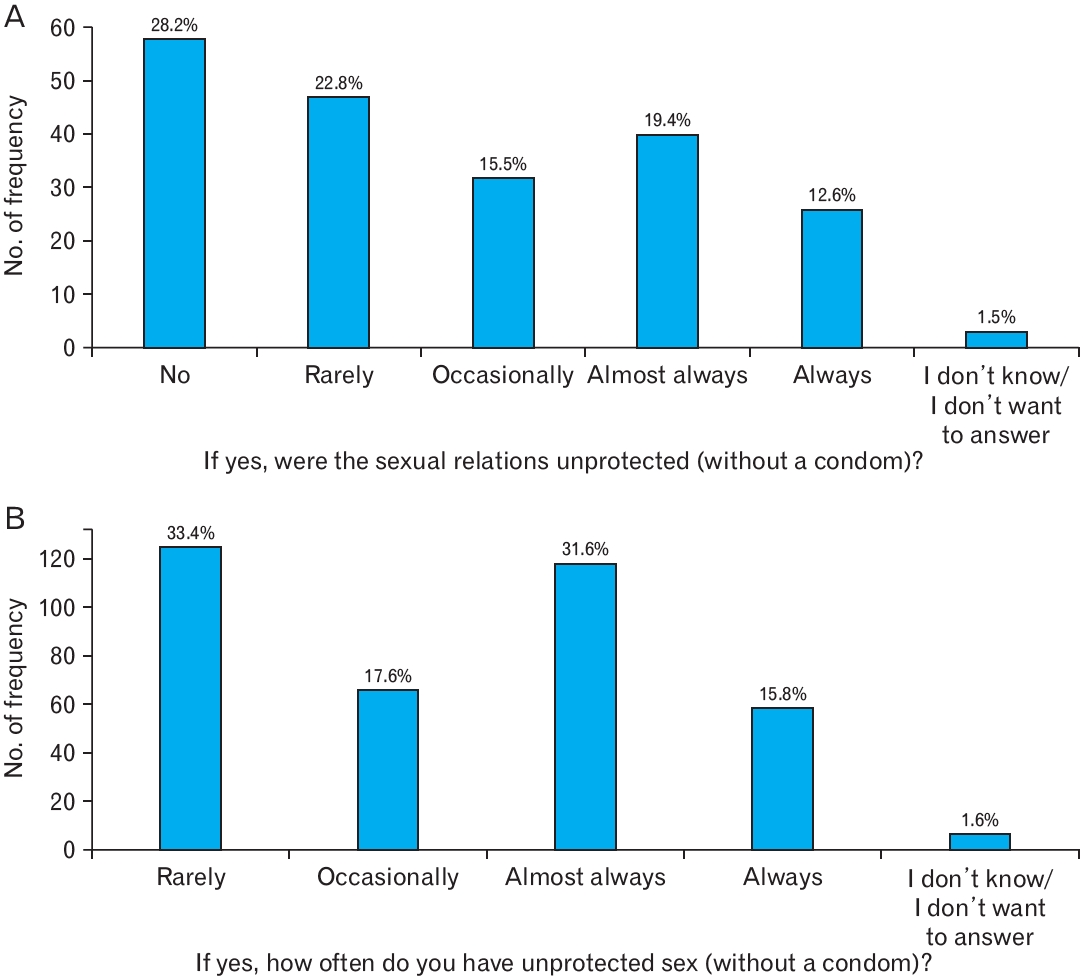
STI contraction was significantly associated with sexual orientation (P<0.001). We found that 22.7% of homosexual individuals contracted STI, as opposed to only 2.5% of heterosexual individuals. The frequency at which students have unprotected sex (without condoms), either without or under the influence of alcohol and/or drugs, is shown in Figure 1A and B, respectively.
Similar to the previous table, we identified the studentsŌĆÖ risk behaviors that predisposed them to parenteral transmission/contraction of HIV infection. No significant differences were found between medical students and those from other healthcare fields. The differences between women and men are presented in Table 6.
Ninety-nine percent of the inquired patients denied intravenous drug use, and the remaining 0.3% did not want to answer the question. Since there were no affirmative answers regarding the consumption of illicit drugs by injection, no answers were obtained for the following questions about this route of transmission. We also quantified the studentsŌĆÖ fear of HIV contraction/transmission using a scale from 1 to 4, with 1 meaning ŌĆ£not afraidŌĆØ and 4 meaning ŌĆ£very afraid,ŌĆØ and compared these results between men and women. The results are presented in Table 7.
HIV continues to be a public health problem; therefore, training and raising awareness among college healthcare students on this subject is extremely important.
This study showed that only 20.1% of the students had ever been tested for HIV. In addition, 59.6% of the students had never been approached by their family doctors about the test or risk behaviors for virus transmission. However, among the students tested for HIV, the main reason for testing was screening (39.0%). Similar studies involving university students support these results, such as those of Nkwonta and Harrison [13] in 2021, in which only 8% of students had ever been tested for HIV, and Lin et al. [14] in 2017, in which most students had never been tested for HIV, despite maintaining risky sexual behaviors.
In Europe, GPs can greatly contribute to the early detection of HIV; however, there are evidences of multiple missed diagnostic opportunities [15]. Several barriers to HIV testing among GPs were identified by Deblonde et al. [15], including lack of sexual health communication skills, lack of knowledge about the screening recommendations and epidemiological specifics of HIV, and lack of experience in communicating the test results.
HIV testing was significantly associated with sexual orientation (P<0.001), particularly in homosexual and bisexual students. In line with the present study, Caldeira et al. [16] in 2012 also concluded that HIV testing is more prevalent among non-heterosexual college students.
In October 2019, HIV self-testing was introduced in Portugal and is currently available in pharmacies. This can be a solution to increase HIV testing, especially for those who wish to do so anonymously, given the stigma associated with testing [17].
In October 2019, HIV self-testing was introduced in Portugal and is currently available in pharmacies. This can be a solution to increase HIV testing, especially for those who wish to do so anonymously, given the stigma associated with testing [17].
This study assessed the knowledge regarding HIV transmission routes in healthcare students who are future healthcare professionals, and also screened these students. Most students had sound knowledge regarding the three main transmission routes, as previously found in other studies involving university students [19-22]. However, medical students had significantly lower knowledge regarding HIV transmission through personal utensils (P=0.041) than students from other healthcare fields. In contrast, knowledge of the increased risk of HIV transmission through oral sex (P=0.035), co-infection by STIs (P=0.004), and sharing needles or other equipment used to prepare illicit drugs for injection (P=0.001) was notably higher in medical students.
In contrast to the studentsŌĆÖ good understanding of the transmission routes, this study showed that 66.3% of students did not know the level A recommendation for HIV screening in adolescents and adults. The proportion of students from other healthcare fields who did not know the screening recommendation level was significantly higher than that of medical students (P=0.010). This fact may explain, to date, the low rates of HIV testing among healthcare students (20.1%) and may put its application to future patients of medical students at risk.
Despite the good general knowledge of the participants of this study, this was not necessarily reflected in good sexual practice. Of the 74.6% of sexually active students, 82.0% reported having or having had unprotected sex (without a condom), a fact corroborated by several studies on college students, including Stutts et al. [20], in which only 24.6% of college students reported using condoms during sexual intercourse. Although 33.4% of students reported that they rarely had unprotected sex, 31.6% and 15.8% of them admitted to having unprotected sex almost always and always, respectively.
Most students (53.3%) had never had sexual intercourse under the influence of alcohol and/or drugs, and of those who had (34.0% under alcohol and 11.4% under both), 28.2% reported that sexual intercourse was not unprotected or that it was rare (22.8%). Thus, there was no increase in the frequency of unprotected sexual intercourse under the influence of alcohol and/or drugs. These results can be explained by Cooper [23], who found that alcohol consumption is significantly associated with an increase in risky sexual behaviors, such as being associated with multiple sexual partners and occasional partners, but is not directly related to an increase in unprotected sexual intercourse. However, other studies defy these conclusions, proving that college students under the influence of alcohol and/or drugs are more likely to have unprotected sexual intercourse [21,24].
The number of sexually active male students was higher than that of female students (P<0.001). Men had a higher number of sexual partners (P<0.001) and a shorter interval between two consecutive partners than women (P<0.001). Men were also more likely to have sex under the influence of alcohol and/or drugs than women (P=0.010). Men practiced more unprotected oral (P<0.001) and anal (P<0.001) sex and more often with occasional partners (P<0.001) than women. Women engaged in significantly more unprotected vaginal sex (P<0.001) and relational partners (P=0.017) than men. Previous studies have also proven that male college students have a higher overall propensity for risky sexual behaviors [25]. According to Munoz-Silva et al. [26] in 2009, female college students have more unprotected sex with relational partners than male students, which is in agreement with the findings of the present study. The low use of condoms can be justified by the results of other studies that have revealed that women in Europe who are sexually active in stable relationships tend to use the pill as a contraceptive method [27].
Only 3.5% of students reported having contracted an STI. Furthermore, the occurrence of these infections was significantly associated with sexual orientation (P<0.001), particularly among homosexual individuals. Shover et al. [28] in 2018 analyzed the disparities between sexual orientation in the incidence of HIV and other STIs. They concluded that the highest incidence of STIs (35.0%) was among homosexual and bisexual males.
None of the inquired participants reported intravenous drug use, and most students (69.9%) never shared personal utensils, which is in agreement with their knowledge about this transmission route. Students who shared personal utensils (29.0%) did so rarely (65.0%) or with family members (72.9%). Even so, in 2021, the European Report on Drugs revealed that Portugal ranks fifteenth in Europe regarding the diagnosis of HIV infection related to injecting drug use, with 1.6 cases per a million inhabitants. [29].
Students had a mean score greater than two on all three questions regarding fear of contracting or transmitting HIV infection sexually and parenterally. This is reflected in the studentsŌĆÖ behaviors towards parenteral transmission but not in their sexual behaviors. In contrast, Inungu et al. [22] in 2009, reported that most students (86.8%) do not consider themselves at risk of HIV infection, which could justify the low rates of HIV testing. In the present study, students showed fear of HIV contraction/transmission, which does not justify the low percentages of HIV testing.
One of the limitations of this study was that the questionnaire was not uniformly disseminated to account for the entire population, both in terms of the study area and different regions of the country.
Students voluntarily participated in this study, which caused a voluntarism bias. ParticipantsŌĆÖ answers may not correspond to their actual behavior, which causes a social desirability bias. Similarly, the assessment of studentsŌĆÖ knowledge was done through simple questions, the answers of which were easily found online; thus, the answers may not correspond to the actual knowledge of the students.
A strength of this study is that 79.5% of the participants were women, a percentage close to the sex distribution of the target population, since approximately 77.7% of students in healthcare courses in Portugal are women [30].
Another aspect worth highlighting is that, throughout the questionnaire, the option ŌĆ£I do not know/I do not want to answerŌĆØ was always present to respect each participantŌĆÖs privacy whenever they did not feel comfortable answering.
To the best of our knowledge, there are no studies on this topic, at least among Portuguese healthcare students, and the present study provides original data on this theme. This study addresses issues that still carry much stigma in society. Given the enormous importance of sexual health, and since HIV is a public health problem, this study may open doors to future studies in this area.
In conclusion, it is essential for GPs and awareness campaigns to alert and disseminate HIV screening tests. Sexual health and risk behaviors should be addressed more frequently in schools to educate healthcare students. It is important to raise awareness among students and future healthcare professionals since prevention is the best strategy against HIV transmission/contraction. GPs can and should play a more active role in disseminating and prescribing HIV screening tests, as well as in alerting individuals to risk behaviors that predispose them to HIV transmission.
Figure.┬Ā1.
(A) Frequency at which students have unprotected sex. (B) Frequency at which students have unprotected sex under the influence of alcohol and/or drugs.
Table┬Ā1.
Descriptive statistics of the sampleŌĆÖs study areas
Table┬Ā2.
HIV screening test and dissemination of information regarding HIV by general practitioners
Table┬Ā3.
HIV screening test among students with different sexual orientations
Table┬Ā4.
Knowledge about HIV transmission and screening among areas of study
Table┬Ā5.
Sexual risk behaviors among students of different genders
Table┬Ā6.
Risk behaviors among students of different genders that predispose to HIV parenteral transmission
Table┬Ā7.
Fear of contracting/transmitting HIV among students of different genders
REFERENCES
1. AIDSinfo. Epidemic & response [Internet]. Geneva: AIDSinfo; 2020 [cited 2021 Oct 8]. Available from: https://aidsinfo.unaids.org/
2. Vidya Vijayan KK, Karthigeyan KP, Tripathi SP, Hanna LE. Pathophysiology of CD4+ T-cell depletion in HIV-1 and HIV-2 infections. Front Immunol 2017;8:580.



3. Centers for Disease Control and Prevention (CDC). Revised surveillance case definition for HIV infection: United States, 2014. MMWR Recomm Rep 2014;63(RR-03):1-10.
4. U.S. Preventive Services Task Force. Published recommendations [Internet]. Rockville (MD): U.S. Preventive Services Task Force; 2017 [cited 2021 Oct 8]. Available from: http://www.uspreventiveservicestaskforce.org
5. Directorate-General of Health. Diagnosis and laboratory screening of human immunodeficiency virus (HIV) infection [Internet]. Lisbon: Directorate-General of Health; 2011 [cited 2021 Oct 9]. Available from: https://www.pnvihsida.dgs.pt/informacao-tecnica-e-cientifica111/normas-de-orientacao-clinica/norma-n-582011-de-28-dez-2011-atualizada-a-10-dez-2014-pdf.aspx
6. Phanuphak N, Gulick RM. HIV treatment and prevention 2019: current standards of care. Curr Opin HIV AIDS 2020;15:4-12.

7. Dybul M, Attoye T, Baptiste S, Cherutich P, Dabis F, Deeks SG, et al. The case for an HIV cure and how to get there. Lancet HIV 2021;8:e51-8.


9. van der Graaf M, Diepersloot RJ. Transmission of human immunodeficiency virus (HIV/HTLV-III/LAV): a review. Infection 1986;14:203-11.



10. Centers for Disease Control and Prevention. Ways HIV can be transmitted [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2021 [cited 2021 Oct 10]. Available from: https://www.cdc.gov/hiv/basics/hiv-transmission/ways-people-get-hiv.html
11. Directorate-General of Health; National Institute of Health Dr. Ricardo Jorge. HIV infection and AIDS in Portugal 2020 [Internet]. Lisbon: DGS/INSA; 2020 [cited 2021 Oct 11]. Available from: https://www.dgs.pt/portal-da-estatistica-da-saude/diretorio-de-informacao/diretorio-de-informacao/por-serie-1199251-pdf.aspx?v=%3d%3dDwAAAB%2bLCAAAAAAABAArySzItzVUy81MsTU1MDAFAHzFEfkPAAAA
12. International Labor Organization; World Health Organization. Joint ILO/WHO guidelines on health services and HIV/AIDS [Internet]. Geneva: International Labor Organization; 2008 [cited 2021 Oct 12] Available from: https://www.ilo.org/wcmsp5/groups/public/---europe/---ro-geneva/---ilo-lisbon/documents/genericdocument/wcms_651177.pdf
13. Nkwonta CA, Harrison SE. HIV knowledge, risk perception, and testing behaviors among college students in South Carolina. J Am Coll Health 2021;1-8.

14. Lin CA, Roy D, Dam L, Coman EN. College students and HIV testing: cognitive, emotional self-efficacy, motivational and communication factors. J Commun Healthc 2017;10:250-9.



15. Deblonde J, Van Beckhoven D, Loos J, Boffin N, Sasse A, Nostlinger C, et al. HIV testing within general practices in Europe: a mixed-methods systematic review. BMC Public Health 2018;18:1191.




16. Caldeira KM, Singer BJ, OŌĆÖGrady KE, Vincent KB, Arria AM. HIV testing in recent college students: prevalence and correlates. AIDS Educ Prev 2012;24:363-76.



17. Augusto GF. HIV self-testing kits enjoy successful launch in Portugal. Lancet Infect Dis 2019;19:1289.


18. Shahar E, Maor C, Moshe-Eilon Y. Medical personnel knowledge and stigmatic attitude toward HIV patients in a high-income country. AIDS Care 2020;32:1023-9.


19. Degroote S, Vogelaers D, Liefhooghe G, Vermeir P, Vandijck DM. Sexual experience and HIV-related knowledge among Belgian university students: a questionnaire study. BMC Res Notes 2014;7:299.




20. Stutts LA, Robinson PA, Witt B, Terrell DF. Lost in translation: college studentsŌĆÖ knowledge of HIV and PrEP in relation to their sexual health behaviors. J Am Coll Health 2022;70:561-7.


21. Heads AM, Dickson JW, Asby AT. Correlates of HIV risk-taking behaviors among African-American college students: HIV knowledge and ethnic identity. J Health Care Poor Underserved 2017;28(2S):155-70.

22. Inungu J, Mumford V, Younis M, Langford S. HIV knowledge, attitudes and practices among college students in the United States. J Health Hum Serv Adm 2009;32:259-77.

23. Cooper ML. Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. J Stud Alcohol Suppl 2002;(14):101-17.


24. Hightow LB, MacDonald PD, Pilcher CD, Kaplan AH, Foust E, Nguyen TQ, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: transmission among college students. J Acquir Immune Defic Syndr 2005;38:531-7.

25. Dekin B. Gender differences in HIV-related self-reported knowledge, attitudes, and behaviors among college students. Am J Prev Med 1996;12(4 Suppl):61-6.


26. Munoz-Silva A, Sanchez-Garcia M, Martins A, Cristina N. Gender differences in HIV-related sexual behavior among college students from Spain and Portugal. Span J Psychol 2009;12:485-95.


27. Martin TC. Contraceptive use patterns among Spanish single youth. Eur J Contracept Reprod Health Care 2005;10:219-28.


28. Shover CL, DeVost MA, Beymer MR, Gorbach PM, Flynn RP, Bolan RK. Using sexual orientation and gender identity to monitor disparities in hiv, sexually transmitted infections, and viral hepatitis. Am J Public Health 2018;108(S4):S277-83.



29. European Monitoring Centre for Drugs and Drug Addiction. European drug report 2021: trends and developments [Internet]. Luxembourg: Publications Office of the European Union; 2021 [cited 2021 Oct 24]. Available from: https://www.emcdda.europa.eu/system/files/publications/13838/2021.2256_PT_03.pdf
30. PORDATA. Female students in % of those enrolled in higher education: total and by area of education and training [Internet]. Lisbon: PORDATA; 2021 [cited 2021 Oct 25]. Available from: https://www.pordata.pt/Portugal/Alunos+do+sexo+feminino+em+percentagem+dos+matriculados+no+ensino+superior+total+e+por+├Īrea+de+educa├¦├Żo+e+forma├¦├Żo+-1051-8515







