 |
 |
- Search
| Korean J Fam Med > Volume 44(3); 2023 > Article |
|
Abstract
Background
This study aimed to improve the clinical course of patients through rapid response by analyzing the characteristics of critically ill patients with confirmed coronavirus disease 2019 (COVID-19) in Busan between December 1, 2020, and December 31, 2021.
Methods
We divided patients diagnosed with COVID-19 into mild-to-moderate and critical groups based on their clinical severity. The critically ill patients were further sub-divided into delta and delta variant non-epidemic group.
Results
The following factors were significantly more frequent in critically ill patients than in patients with mild-tomoderate symptoms: male sex, age Ōēź60 years, symptoms at the time of diagnosis, and those with underlying diseases. The following factors were significantly more common in the non-delta variant epidemic group than in the delta variant epidemic group in critically ill patients: male sex, age Ōēź60 years, underlying diseases, and not being vaccinated. In the delta variant epidemic group, the duration between confirmation of disease and its progression to critically ill status was significantly shorter than that in the non-delta variant epidemic group.
Since Wuhan City, Hubei Province, China, reported the first case of coronavirus disease 2019 (COVID-19) at the end of December 2019 [1], the number of new weekly cases reported worldwide peaked in March 2022 [2]. The first case of COVID-19 in Busan, a region located on the southeast coast of the Republic of Korea, was reported on February 21, 2020. In May 2020, a hospital dedicated to COVID-19 was established. At the beginning of the outbreak, all patients, including those with mild clinical symptoms, were isolated and treated in a hospital dedicated to infectious diseases. In December 2020, a residential treatment center that could accommodate patients with mild symptoms was opened, and the Busan Infectious Disease Response Team started to allocate beds according to clinical severity, as the bed utilization rate increased. As the daily number of new confirmed cases increased and the number of cases with deteriorating conditions increased, it became important to prepare beds to accommodate critically ill patients and treat them in a timely manner. Therefore, the number of critically ill patients and the severity of COVID-19 are important indicators for establishing response strategies. The incidence of confirmed cases and the availability of intensive care beds differ by region; consequently, hence, it is necessary to study the epidemiological characteristics of critically ill patients in Busan City.
Therefore, in this study, patients with confirmed COVID-19 status in Busan were classified as critical and mild-to-moderately ill patients for a period of 13 months between December 1, 2020, and December 31, 2021, and the characteristics of these groups were comparatively analyzed. We expect the findings of this study to be resourceful in the management and treatment of critically ill patients by comparatively analyzing the differences between the groups according to the prevalence of the delta variant virus in the population.
Patients with confirmed COVID-19 in Busan and those who were treated in the intensive care unit (ICU) after becoming critically ill at hospital dedicated to infectious diseases from December 1, 2020, to December 31, 2021, were included in this study. Patients with COVID-19 are divided into the following four stages according to disease severity: asymptomatic, mild, severe, and critical. Mild disease is characterized by mild symptoms without pneumonia. Severe disease is characterized by dyspnea, tachypnea (>30 breaths per minute), arterial oxygen saturation Ōēż93%, the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) <300, and worsening of pneumonia by >50% within 24ŌĆō48 hours. Critical disease is characterized by respiratory failure, septic shock, and multiple organ failure. The definition of clinical severity has changed since the beginning of the outbreak of COVID-19. In this study, ŌĆ£critically illŌĆØ patients were defined as those who received non-invasive high-flow oxygen therapy, invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO), or continuous renal replacement therapy (CRRT). Patients with mild-to-moderate symptoms were defined as those who received low-dose oxygen therapy at less than 5 L/min at home, a residential treatment center, or a hospital for infectious diseases [3].
The method for confirming COVID-19 is described in the guidelines of the Korea Centers for Disease Control and Prevention (KCDC; currently, Korea Disease Control and Prevention Agency). The specimens were transferred to an in-house laboratory, a private testing institution, or the Busan Health and Environment Research Institute and tested using real-time reverse transcription polymerase chain reaction [4]. Patient epidemiological information was collected using a COVID-19 basic epidemiologic survey via telephone interviews with patients confirmed with COVID-19 at 16 public health centers in Busan. In the basic epidemiological survey, age, sex, underlying diseases, symptoms, and infection route were included. Analysis of variant virus was conducted by the KCDC, and the results were collected by Busan City. The hospitalization and discharge status of patients who deteriorated to critically ill status were obtained from the infection control offices at infectious disease hospitals.
The characteristics of all critically ill patients and those with mild-tomoderate illness were compared and summarized (Table 1). The presence of symptoms at the time of confirmation of disease was determined according to the COVID-19 Basic Epidemiologic Investigation; consequently, the symptoms that followed after confirmation were not included. The differences in the distribution of symptoms at the time of diagnosis between the two groups (Figure 1) and between patients aged up to and beyond 60 years were visualized using a forest plot (Figure 2A, B). The benchmark for determining the prevalence of the delta variant virus is based on the first introduction of the variant virus into Korea on April 22, 2021, and July 2021, when the delta variant virus began to spread [5]. For COVID-19 cases confirmed before July, the KCDC requested variant analysis and mutated gene test and were assigned to the non-epidemic group of the delta variant virus. Delta variant-related cases transmitted by contact were included in the delta variant epidemic group. Investigation of the source of infection based on the route of infection implies that contact history with any previously confirmed person is unknown; among them, those who were tested because they had symptoms, or were those who were workers in facilities vulnerable to infection and who performed periodic preemptive tests, and those who were tested by themselves were included. Patients were included if they had contact at multi-use facilities(restaurants, coffee shops, sports facilities, and entertainment facilities), used such facilities, or if they were tested for a group outbreak at such facilities.
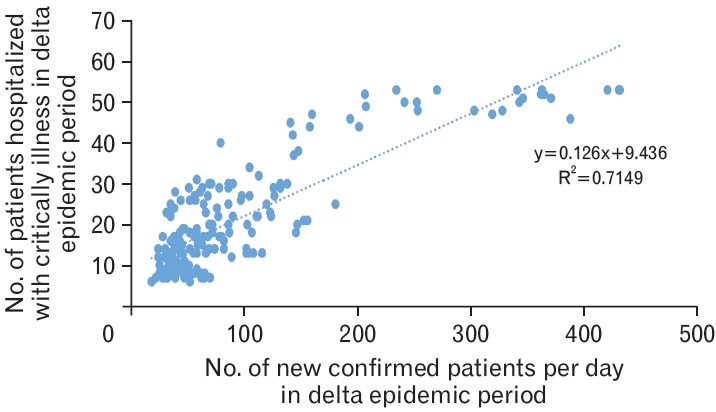
The differences in the characteristics between the delta variant virus epidemic group and the non-epidemic group observed in 460 patients whose symptoms progressed to the point of being critically ill is summarized in Table 2. The number of days critically ill patients were hospitalized in the ICU for was determined by the duration of treatment with non-invasive high-flow oxygen therapy, ventilator, ECMO, or CRRT. Of the 460 patients with critically ill status, 375 patients complained of symptoms at the time of COVID-19 confirmation; the analysis of symptomatic patients from the onset of symptoms to critically ill status was determined from the onset of symptoms to the date of starting treatment as a critically ill patient during hospitalization. Additionally, based on the prevalence of the delta variant virus, the correlation between the number of daily new confirmed cases of COVID-19 and the number of daily hospitalized patients in ICUs during was reported during the period of this study (Figure 3). We analyzed the correlation between the daily number of new confirmed cases and the number of hospitalized patients with critically ill status during the delta variant virus non-epidemic and epidemic period, which revealed that the average duration between confirmation of COVID-19 status and critically ill status was 7.46 days and 4.67 days, respectively (Figures 4, 5). For categorical variables, frequencies and percentages were calculated, and for continuous variables, mean and standard deviation were calculated. In order to compare the difference between patients with critically ill status with and without the delta variant virus, the chi-square test was used; continuous variables were tested using the F test and equal variances (Levene t-test) or heterovariance t-test (WelchŌĆÖs t-test). A simple regression analysis was performed to estimate the correlation coefficient between continuous variables. Statistical significance was set at P<0.05. The statistical program used was SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
This study includes the results of an epidemiological investigation conducted by the national and local health authorities directly in accordance with the ŌĆśLaw on the Prevention and Management of Infectious DiseasesŌĆÖ in a situation that requires urgent action for public health. This falls under Article 33(3) of the Enforcement Rules, and deliberations were not carried out because they were not subject to the institutional review board deliberations.
Between December 1, 2020, and December 31, 2021, there were 24,441 confirmed cases of COVID-19 in Busan. Of them, 460 were hospitalized and treated in the ICU, including patients who were asymptomatic at the time of confirmation, whereas 23,981 patients were treated in the general wards. In critically ill patients, 55% were men, 68.9% were aged Ōēź60 years, 83.7% complained of clinical symptoms at the time of confirmation, and 61.5% had underlying diseases, which was significantly higher than the proportion in patients with mild-to-moderate disease severity (P<0.001). The underlying diseases in critically ill patients included hypertension (n=107 [23.3%]), diabetes (n=73 [15.9%]), and hyperlipidemia (n=23 [5%]); while those in patients with mild-tomoderate disease included hypertension (n=2,739 [11.4%]), diabetes (n=1,647 [6.9%]), and hyperlipidemia (n=858 [3.8%]). Additionally, other diseases in critically ill patients included cardiovascular disease excluding hypertension (n=71 [15.4%]), chronic respiratory diseases including asthma (n=37 [8%]), dementia including AlzheimerŌĆÖs and ParkinsonŌĆÖs dementia (n=33 [7.2%]), cerebrovascular disease including cerebral infarction and cerebral hemorrhage (n=23 [5%]), and cancer (n=21 [4.6%]). Other diseases in patients with mild-to-moderate disease included cerebrovascular disease including cerebral infarction and cerebral hemorrhage (n=243 [3.6%]), cardiovascular disease excluding hypertension (n=643 [2.7%]), chronic respiratory disease including asthma (n=535 [2.2%]), and cancer (n=512 [2.1%]). In terms of the distribution of symptoms according to disease severity, there was no significant difference in the prevalence of hyperlipidemia and thyroid disease between patients with critical and mild-to-moderate illness (P=0.10 and P=0.73, respectively). The prevalence rates of all other underlying diseases were significantly higher in critically ill patients (P<0.001). In patients with mild-to-moderate disease, the estimated path of the infection was found to be high in 74.9% of cases (P<0.001); there was no significant difference in the prevalence of the delta variant virus between the two groups (P=0.06) (Table 1).
We analyzed the differences in the distribution of symptoms at the time of confirmation of COVID-19 in 375 critically ill patients and 17,416 patients with mild-to-moderate disease. The rates of dyspnea (odds ratio [OR], 10.84; 95% confidence interval [CI], 8.33ŌĆō14.11; P<0.001), chest discomfort (OR, 3.8; 95% CI, 2.27ŌĆō6.37; P<0.001), fever (OR, 2.71; 95% CI, 2.13ŌĆō3.44; P<0.001), dizziness (OR, 2.58; 95% CI, 1.36ŌĆō4.89; P<0.05), loss of taste (OR, 1.86; 95% Cl, 1.28ŌĆō2.69; P<0.001), sputum (OR, 1.83; 95% CI, 1.46ŌĆō2.30; P<0.001), myalgia (OR, 1.67; 95% CI, 1.33ŌĆō2.10; P<0.001), chills (OR, 1.57; 95% CI, 1.19ŌĆō2.06; P<0.05), and cough (OR, 1.39; 95% CI, 1.14ŌĆō1.69; P<0.01) were higher in critically ill patients than those in patients with mild-to-moderate symptoms. In contrast, the rates of a runny nose (OR, 0.42; 95% CI, 0.25ŌĆō0.69; P<0.001), sore throat (OR, 0.75; 95% CI, 0.58ŌĆō0.96; P<0.05), and nasal congestion (OR, 0.29; 95% CI, 0.12ŌĆō0.71; P<0.01) were significantly lower in critically ill patients than those in patients with mild-to-moderate symptoms. The rates of fatigue (OR, 1.50; 95% CI, 0.55ŌĆō4.08; P=0.42), fever (OR, 1.25; 95% CI, 0.97ŌĆō1.62; P=0.08), diarrhea (OR, 1.21; 95% CI, 0.49ŌĆō2.95; P=0.68), headache (OR, 0.84; 95% CI, 0.63ŌĆō1.12; P=0.24), and loss of smell (OR, 0.88; 95% CI, 0.55ŌĆō1.40; P=0.59) were not significantly different between the groups (Figure 1). Additionally, the distribution of the symptoms was analyzed by dividing groups of patients into those <60 years old and Ōēź60 years old. In patients aged <60 years, the rates of cough (OR, 1.47; 95% CI, 1.03ŌĆō2.09; P<0.001), sputum (OR, 1.84; 95% CI, 1.23ŌĆō2.75; P<0.001), dyspnea (OR, 14.7; 95% CI, 9.44ŌĆō22.89; P<0.001) were significantly lower in critically ill patients, while chest discomfort (OR, 2.97; 95% CI, 1.08ŌĆō1.88; P<0.001), myalgia (OR, 2.14; 95% CI, 1.47ŌĆō3.13; P<0.001), chills (OR, 2.32; 95% CI, 1.41ŌĆō 3.56; P<0.001), fever (OR, 2.89; 95% CI, 1.95ŌĆō4.28; P<0.001), and loss of taste (OR, 2.65; 95% CI, 1.57ŌĆō4.49; P<0.001) were significantly higher in critically ill patients than the rates in those with mild-to-moderate disease. Furthermore, the rate of a runny nose (OR, 0.23; 95% CI, 0.07ŌĆō 0.72; P<0.001) was significantly lower in the critically ill group compared to that in the mild-to-moderate group (Figure 2A).
In patients aged Ōēź60 years, the rates of sputum (OR, 1.96; 95% CI, 1.47ŌĆō2.61; P<0.001), dyspnea (OR, 7.18; 95% CI, 5.13ŌĆō10.05; P<0.001), chest discomfort (OR, 2.45; 95% CI, 1.12ŌĆō5.39; P<0.001), fever (OR, 4.54; 95% CI, 3.30ŌĆō6.25; P<0.001), mild fever (OR, 1.97; 95% CI, 1.41ŌĆō 2.75; P<0.001), and loss of taste (OR, 2.51; 95% CI, 1.45ŌĆō4.34; P<0.001) were significantly higher in critically ill patients, while the rate of a runny nose (OR, 0.49; 95% CI, 0.25ŌĆō0.93; P=0.03) was significantly lower in critically ill patients in comparison with the rates in patients with mildto-moderate symptoms (Figure 2B).
In the delta variant non-epidemic group, 92.1% of the patients were aged >60 years, 77.4% had underlying diseases, 73% had an identified source of infection, and 98.3% were not vaccinated (Table 2). In contrast, in the delta variant epidemic group, 61.5% were aged >60 years, 51% had underlying diseases, 50.1% had an identified source of infection, and 57.7% were not vaccinated (P<0.001, respectively). Between the delta non-epidemic and epidemic groups, hypertension was the most common underlying disease (n=32 [27.8%] and n=82 [23.7%], respectively), followed by diabetes (n=21, [18.3%] and n=8 [2.3%], respectively), with no significant differences (P>0.05 for both). Additionally, the prevalence rates of chronic respiratory diseases, including hyperlipidemia and asthma, dementia, including AlzheimerŌĆÖs and ParkinsonŌĆÖs dementia, and cerebrovascular diseases, including cerebral infarction and cerebral hemorrhage, were significantly higher in the delta variant non-epidemic group than those in the delta variant epidemic group (P<0.001). The number of patients with symptoms at the time of COVID-19 confirmation was significantly higher in the delta variant epidemic group (88.4%) than those (60.9%) in the non-epidemic group (P<0.001). Furthermore, there was no difference in the sex distribution between the groups (P=0.63). There was no significant difference in the duration between the onset of symptoms and their progression to critically ill (P=0.17). The average age in the delta variant non-epidemic group was 74.5 years, which was significantly higher than those in the epidemic group (62.9 years) (P<0.05). Additionally, in the delta variant epidemic group, the duration of ICU stay was 11.5 days, and the duration between confirmation of disease and progression to critically ill status was 4.67 days, which were both significantly shorter than those in the non-epidemic group (16 days and 7.46 days, respectively; P<0.05).
The graph of the monthly incidence of newly confirmed cases of COVID-19 and the daily number of hospitalized patients with critical illness revealed that the concordance rate increased from mid-2021 (Figure 3). In the delta variant non-epidemic group, the correlation coefficient (r) was 0.0473 between the number of daily new confirmed cases and the number of hospitalized critically ill patients, while the coefficient of determination (R2) was 0.0022 (P=0.5), thus indicating no statistical significance (Figure 4).
In the delta variant epidemic group, the correlation coefficient (r) between the number of daily new confirmed cases and the number of hospitalized critically ill patients was very high (0.83), and the coefficient of determination (R2) was 0.7149. Consequently, the regression equation was ŌĆ£y=0.126x+9.436ŌĆØ, which indicated statistical significance (P<0.05) (Figure 5).
Richardson et al. [6] reported that 14.2% of 5,700 patients admitted to hospitals in New York City, USA, received intensive care. In the early phase of the COVID-19 outbreak in China, approximately 20% of patients diagnosed with COVID-19 experienced severe illness and required hospitalization; another study reported that a quarter of hospitalized patients needed treatment in the ICU [7]. Between December 1, 2020, and December 31, 2021, 24,441 patients had confirmed COVID-19 in Busan; of them, 460 (1.9%) were classified as critically ill. In a study that analyzed the epidemiologic characteristics of critically ill patients with COVID-19, the incidence of critically ill status was found to be 52%ŌĆō84% more frequent in men than in women [8]. The average age of critically ill patients was 62ŌĆō70 years, with older patients more frequently affected than the younger ones [9]. In a Swiss study, the average age of critically ill and non-critically ill patients was 62.4 years and 46 years, respectively. Similarly, we observed a significantly higher frequency of males and patients aged >60 years in the critically ill group than those in the mild-to-moderate group.
According to a Korean study, the most prevalent symptoms of patients with COVID-19 included cough (n=2,247 [42.0%]), sputum (1,563 [29.2%]), temperature >37.5┬░C (n=1,249 [23.3%]), and headache (n=946 [17.7%]) [10]. In our study, the most common symptoms included cough (n=6,167 [18%]), sore throat (n=5,100 [14.9%]), myalgia (n=3,362 [9.8%]), headache (n=3,263 [9.5%]), and fever (5.7%). A Swiss study confirmed that dyspnea was the only high-risk symptom in critically ill patients (OR, 6.42; 95% CI, 2.44ŌĆō16.9) [11].However, in our study, dyspnea was 11 times more common in critically ill patients than in patients with mild-to-moderate disease, while dizziness and loss of taste, in addition to chest discomfort and fever, were 2ŌĆō3 times more likely in patients with mild-to-moderate disease than those in patients with critically ill status. The fact that the symptoms were significantly higher should be considered when assigning a bed at the time of diagnosis, and additional research on this topic is needed. In our study, sputum, dyspnea, chest discomfort, fever, and loss of taste occurred more frequently in critically ill patients than in patients with mild-tomoderate symptoms irrespective of the age group. Therefore, it may be crucial to recognize these symptoms at the time of confirmation of COVID-19 as indicators of progression towards severe illness. However, the major difference is that the frequencies of cough, muscle pain, and chills, which were common in critically ill patients <60 years of age, were not significantly high in critically ill patients Ōēź60 years of age. Additionally, loss of taste, irrespective of age, is a frequent symptom in critically ill patients and is considered one of the main factors for hospitalization for COVID-19 when fever or shortness of breath persists; therefore, it is a symptom to be aware of.
Among the underlying diseases in critically ill patients with COVID-19, chronic obstructive pulmonary disease (OR, 6.42; 95% CI, 2.44ŌĆō 16.9) and cardiovascular diseases including hypertension (OR, 17.8; 95% CI, 6.56ŌĆō48.2) were highly prevalent [12]. It was reported that 58% of critically ill patients had diabetes [13]. In this study, high blood pressure and diabetes were common diseases in critically ill patients with COVID-19. The prevalence rates of cardiovascular diseases, excluding hypertension, and chronic respiratory diseases including asthma and chronic bronchitis, were significantly higher in critically ill patients than those in patients with mild-to-moderate disease. In one study, the average period of ICU hospitalization in critically ill patients with COVID-19 was 9ŌĆō18 days [14]. In this study, the average duration was 12.6┬▒12.2 days and the median duration was 9 days, which is similar to the previous findings. In China, patients were generally transferred to the ICU approximately 10 days after the onset of symptoms [15]. However, in this study, the duration of hospitalization in the delta variant non-epidemic group was 8.41 days and that in the epidemic group was 9.6 days, with no statistically significant difference based on the prevalence of the delta variant virus. Mortality in ICU patients due to COVID-19 varied from 16% to 78% [15-18]. In this study, the fatality rate among 113 critically ill patients was 24.6%.
In patients with confirmed delta variant virus COVID-19 infection, the severity was worse than that in patients with non-variants or the alpha variant. In a Canadian study, the risk of hospitalization, admission to the ICU, and death was 120%, 287%, and 137% higher, respectively, in the delta variant group than that in the non-variant group [19]. Additionally, in a survey of emergency room admissions in the United Kingdom, the risk of hospitalization or emergency room admission within 14 days of infection with the delta variant was 1.45 times higher than that after infection with the alpha variant [20]. In this study, the duration between the time of diagnosis and exacerbation of severe symptoms was significantly shorter in the delta variant epidemic group than that in the non-epidemic group, which indicates that the conversion rate to critical severity in delta variant infection is rapid. Furthermore, we found that there was no significant difference in the incidence of critically ill status between those with and without the delta variant virus. In Busan, vaccination against COVID-19 infection was implemented in February 2021. On July 1, 2021, the proportion of people who had their first vaccination was 33%, while those who received their second vaccination was 10%. The primary inoculation rate was 80%, and the secondary inoculation rate was 75%. Studies have demonstrated that the existing delta variant causes more severe disease than the non-variants or the alpha variants [19,20]. A study in India revealed that the number of critically ill patients in the unvaccinated group was 54.1%, which was significantly higher than (30.3%) in the vaccinated group [21]. Consequently, vaccination helped in preventing the severity of COVID-19 caused by the delta variant virus.
During the variant epidemic, we compared some of the epidemiological characteristics of critically ill patients with COVID-19 in Busan City and the country. Before the emergence of the delta variant, the proportion of people over 60 years of age was 74.5% (average age, 67.7┬▒13.7 years) nationwide and 92.1% (average age, 74.5┬▒11.7 years) in Busan. During the period of delta variant infection, the proportion of people over 60 years of age was 71.4% (average age, 66.0┬▒15.4 years) nationwide and 61.5% (average age, 62.8┬▒15.4 years) in Busan. In terms of sex distribution, before the emergence of the delta variant, men accounted for 59.8% of all cases in the country and 57.4% in Busan. During the period of delta variant prevalence, men accounted for 57.6% of cases in the nation and 54.8% in Busan. In terms of the distribution of unvaccinated people, during the period when the delta strain was not prevalent, the proportion of people who had not been vaccinated was 97.3% nationwide and 98.3% in Busan, and during the period when the delta variant was prevalent, the proportion of people who had not been vaccinated was 60.6% nationwide and 57.7% in Busan [22].
In Busan, during the period of this study, the correlation coefficient between the number of daily new confirmed cases of COVID-19 and the number of critically ill patients who were hospitalized during the delta variant virus epidemic was as high as 8. If the number of beds required to treat critically ill patients can be predicted, subsequently medical care can improve. If a patient has a stable breathing pattern and their oxygen saturation decreases in room air, the first step is to treat with a nasal cannula or nasal prongs with a low concentration of oxygen (1ŌĆō3 L/min). Secondly, oxygen masks (simple facial masks or O2 masks) with a capacity of 100ŌĆō200 mL can be used for patients who require oxygen at higher concentrations (5ŌĆō10 L/min) than those supplied by the nasal cannula. A reserve bag can be attached to treat emergency cases that require a very high concentration of oxygen (Ōēź10 L/min). In the third step, devices that continuously supply of oxygen, such as a high-flow nasal cannula or optiflow can be used for a high flow rate of 60 L/min irrespective of the patientŌĆÖs breathing pattern [22]. High-flow oxygen therapy, mechanical ventilation, and ECMO can be used for critically ill patients who meet the following criterion for admission to the ICU: ŌĆ£A person who needs or is expected to need more than the supply of invasive mechanical ventilation.ŌĆØ In addition to those receiving ECMO and CRRT, those receiving oxygen over 5 L/min are included [3,23]. The concept of ŌĆ£critically illŌĆØ patients refers to those who are admitted to the ICU and need high-flow oxygen equipment with an oxygen supply of at least 5 L/min. Patients treated in the ICU with oxygen levels of 5ŌĆō15 L/min are excluded by the criterion, and they should be quickly switched to high-flow oxygen therapy or ventilator therapy if oxygen saturation decreases. Therefore, it is important to establish the criteria that consider the difference between critically ill patients and those admitted to the ICU in terms of medical resource management, beds, and specialized medical personnel.
Lastly, the COVID-19 severity criteria should exclude the need for severe respiratory equipment (high-flow oxygen devices, ventilators, ECMOs, and CRRTs) since such equipment is limited per hospital. Instead, reestablishing the ŌĆ£ICU incidence rate,ŌĆØ which is also part of the ICU admission criteria in COVID-19, as a case of requiring oxygen at a rate of 5 L/min or more based on O2 masks, used in university as well as nursing hospitals, will help manage patients and hospital resources efficiently. Coronavirus undergoes mutations periodically, and as a result of repeated epidemics, intensive management of those with critically ill status is necessary. An efficient and sustainable plan should be established with the concept of ŌĆ£with COVID in terms of medical responseŌĆØ by analyzing the current situation.
A limitation of this study is that the period of this study was between December 1, 2020, and December 31, 2021. Therefore, the data regarding the omicron variant is not included in this analysis, and the analyses included data preceding and following the delta variant epidemic. Given that this was a cross-sectional study, the correlation was identified, but a causal relationship could not be established. Among the underlying diseases, the body mass index (BMI) was not included as a variable because the BMI was not recorded in the basic epidemiological survey. However, the significance of this study is that it can lead to practical changes that can improve the clinical course of patients through rapid response and intensive care management by identifying the characteristics of patients admitted to the ICU with a worse severity of COVID-19.
Figure.┬Ā1.
Forest plot showing the differences in the distribution of symptoms at the time of confirmation of disease according to critical illness of patients with coronavirus disease 2019 (COVID-19) in Busan (n=24,441). Odds ratio, confidence interval, and P-value were obtained using the following website: https://www.medcalc.org/calc/odds_ratio.php.
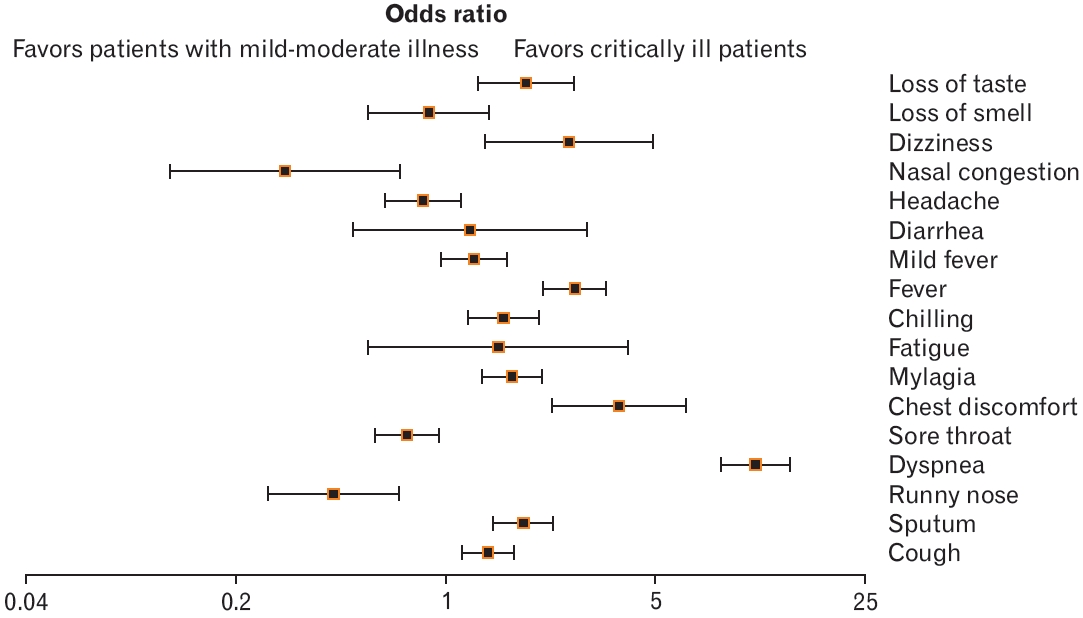
Figure.┬Ā2.
Forest plot showing the differences in the distribution of symptoms at the time of confirmation of disease according to critical illness of patients younger than 60 years of age (A) and patients older than the age of 60 years (B). Odd ratio, confidence interval, and P-value were obtained using the following website: https://www.medcalc.org/calc/odds_ratio.php.
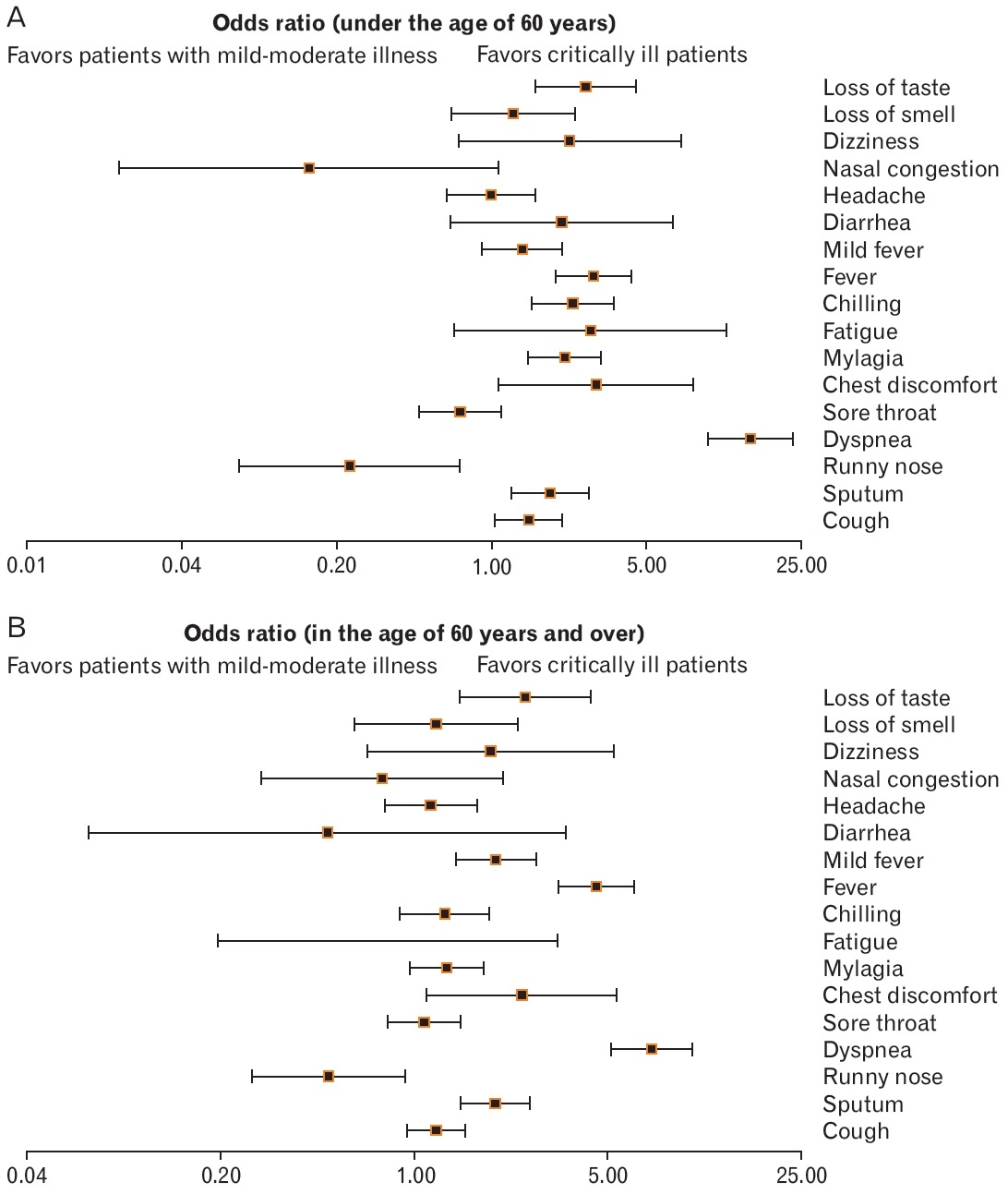
Figure.┬Ā3.
Newly confirmed cases and patients hospitalized with critical illness in Busan. These data do not reflect the period between the date of confirmation and becoming critically ill. *The non-delta epidemic period is before July 2021, when the delta variant began to emerge. However, three cases of delta variants are excluded from the analysis.
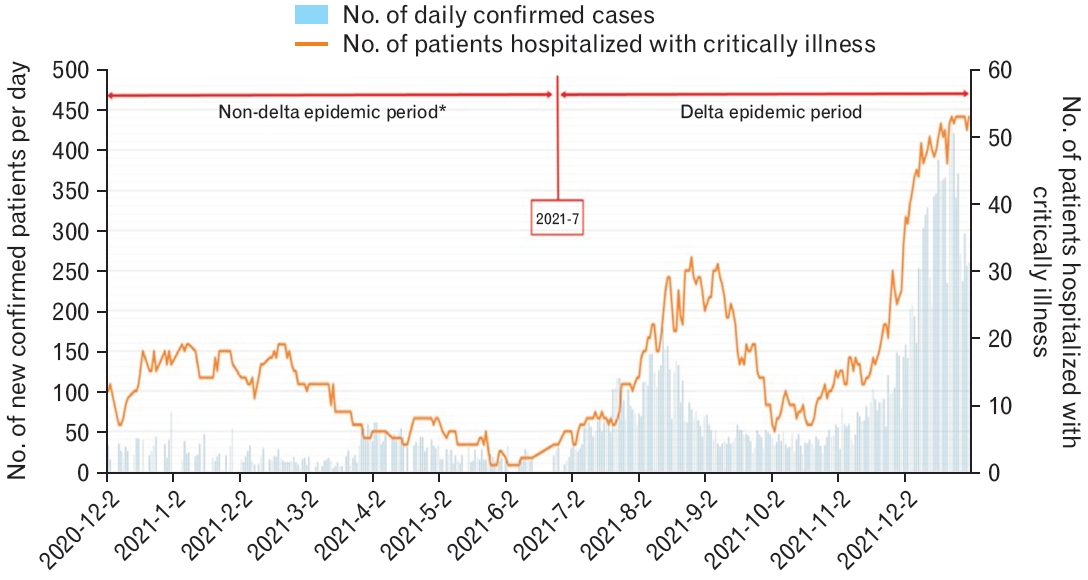
Figure.┬Ā4.
Association between the number of newly confirmed cases of coronavirus disease 2019 (COVID-19) and the number of patients with COVID-19 hospitalized for critical illness in the non-delta epidemic period. The above analysis was conducted to reflect that during the non-delta epidemic period, the average duration between the time of confirmation and becoming critically ill was 7.46 days (Table 2). This result is derived using correlation analysis and simple linear regression (P=0.56).
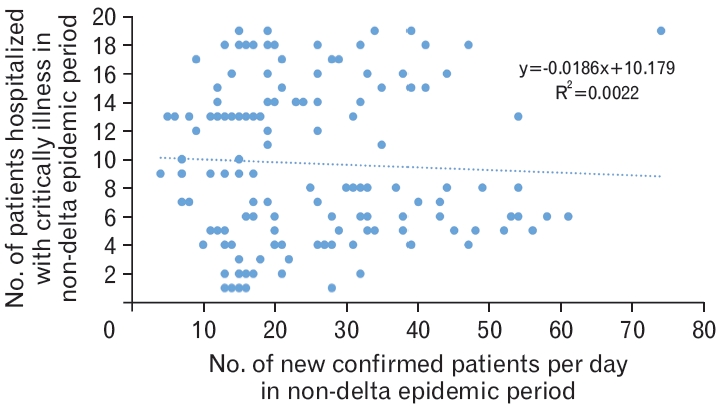
Figure.┬Ā5.
Association between the number of newly confirmed cases of coronavirus disease 2019 (COVID-19) and the number of patients with COVID-19 hospitalized with critical illness in the delta epidemic period. The above analysis was conducted to reflect that during the delta epidemic period, the average duration between the time of confirmation and becoming critically ill was 4.67 days (Table 2). This result is derived using correlation analysis and simple linear regression (P<0.001).
Table┬Ā1.
General characteristics of 24,441 COVID-19 patients in Busan from December 1, 2020 to December 31, 2021 according to whether or not condition of critically illness
| Characteristic | Critically ill patients (n=460) | Patients with mild-moderate illness (n=23,981) | P-value | |
|---|---|---|---|---|
| Sex | 0.013 | |||
| Female | 207 (45.0) | 12,187 (50.8) | ||
| Male | 253 (55.0) | 11,794 (49.2) | ||
| Age group (y) | <0.001 | |||
| <60 | 143 (31.1) | 16,857 (70.3) | ||
| Ōēź60 | 317 (68.9) | 7,124 (29.7) | ||
| Symptoms at the time of confirmation | <0.001 | |||
| Non-existence | 75 (16.3) | 6,565 (27.4) | ||
| Existence | 385 (83.7)) | 17,416 (72.6) | ||
| Underlying disease | <0.001 | |||
| Non-existence | 177 (38.5) | 15,968 (66.6) | ||
| Existence | 283 (61.5) | 8,013 (33.4) | ||
| Hypertension | 107 (23.2) | 2,739 (11.4) | <0.001 | |
| Diabetes mellitus | 73 (15.9) | 1,687 (7.0) | <0.001 | |
| Hyperlipidemia | 23 (5) | 858 (3.8) | 0.10 | |
| Cardiovascular disease* | 71 (15.4) | 643 (2.7) | <0.001 | |
| Chronic respiratory diseaseŌĆĀ | 37 (8.0) | 535 (2.2) | <0.001 | |
| DementiaŌĆĪ | 33 (7.2) | 477 (2.0) | <0.001 | |
| Cerebrovascular disease┬¦ | 23 (5.0) | 243 (3.6) | <0.001 | |
| Cancer | 21 (4.6) | 512 (2.1) | <0.001 | |
| Thyroid disease | 9 (2.0) | 418 (1.7) | 0.73 | |
| Renal failure | 3 (0.9) | 39 (0.2) | <0.001 | |
| Delta variant | 0.06 | |||
| Non-epidemic | 118 (25.7) | 5,274 (22.0) | ||
| Epidemic | 342 (74.3) | 18,707 (78.0) | ||
| Estimated path of the infection | <0.001 | |||
| Unknown | 203 (44.1) | 6,010 (25.1) | ||
| Known (listed below by type) | 257 (55.9) | 17,971 (74.9) | ||
| Family related | 91 (19.8) | 6,824 (28.5) | ||
| Contacts in multi-use facilities | 135 (29.3) | 6,966 (29.0) | ||
| Workplace related | 15 (3.3) | 1,868 (7.8) | ||
| Friend related | 14 (3.0) | 1,935 (8.1) | ||
| Imported case | 2 (0.4) | 378 (1.6) | ||
Table┬Ā2.
General characteristics of 460 critically ill COVID-19 patients in Busan according to whether or not group of delta variant are epidemic
| Characteristic | Delta variant non-epidemic group (n=115) | Delta variant epidemic group (n=345) | P-value | |
|---|---|---|---|---|
| Sex | 0.63* | |||
| Female | 49 (42.6) | 156 (45.2) | ||
| Male | 66 (57.4) | 189 (54.8) | ||
| Age group (y) | <0.001* | |||
| <60 | 9 (7.8) | 133 (38.6) | ||
| Ōēź60 | 106 (92.1) | 212 (61.5) | ||
| Symptoms at the time of confirmation | <0.001* | |||
| Non-existence | 45 (39.1) | 40 (11.6) | ||
| Existence | 70 (60.9) | 305 (88.4) | ||
| Underlying disease | <0.001* | |||
| Non-existence | 26 (22.6) | 169 (49.0) | ||
| Existence | 89 (77.4) | 176 (51.0) | ||
| Hypertension | 32 (27.8) | 82 (23.7) | 0.81* | |
| Diabetes mellitus | 21 (18.3) | 8 (2.3) | 0.39* | |
| Hyperlipidemia | 3 (2.6) | 1 (0.3) | 0.02* | |
| Cardiovascular diseaseŌĆĀ | 9 (7.8) | 23 (6.7) | 0.67* | |
| Chronic respiratory diseaseŌĆĪ | 20 (17.4) | 19 (5.5) | <0.001* | |
| Dementia┬¦ | 21 (18.3) | 12 (3.5) | <0.001* | |
| Cerebrovascular diseaseŌłź | 18 (15.7) | 6 (1.7) | <0.001* | |
| Cancer | 7 (6.1) | 15 (4.3) | 0.45* | |
| Thyroid disease | 2 (1.7) | 7 (2.0) | 0.85* | |
| Renal disease | 1 (0.9) | 3 (0.9) | 1* | |
| Estimated path of the infection | <0.001* | |||
| Unknown | 31 (27.0) | 172 (49.9) | ||
| Known (listed below by type) | 84 (73.0) | 173 (50.1) | ||
| Family related | 31 (26.9) | 73 (21.2) | ||
| Contacts in multi-use facilities | 19 (16.5) | 73 (21.2) | ||
| Workplace related | 60 (45.2) | 13 (3.8) | ||
| Friend related | 3 (2.6) | 12 (3.5) | ||
| Imported case | 0 | 2 (0.6) | ||
| Vaccination history | <0.001* | |||
| Vaccination (1st or 2nd) | 2 (1.7) | 146 (42.3) | ||
| Unvaccinated | 113 (98.3) | 199 (57.7) | ||
| Mean age (y) | 74.5┬▒11.7 | 62.8┬▒15.4 | <0.05┬Č | |
| Average of length of stay in the intensive care unit of critically ill patients (d) | 16 | 11.5 | <0.05# | |
| Average of period from the onset of symptom to becoming critically ill patients** (d) | 9.6 | 8.41 | 0.17# | |
| Average of period from the time of confirmation to becoming critically ill patients (d) | 7.46 | 4.67 | <0.05# | |
REFERENCES
1. Lee SW, Yuh WT, Yang JM, Cho YS, Yoo IK, Koh HY, et al. Nationwide results of COVID-19 contact tracing in South Korea: individual participant data from an epidemiological survey. JMIR Med Inform 2020;8:e20992.



2. World Health Organization. Weekly epidemiological update on COVID-19 [Internet]. Geneva: World Health Organization; 2022 [cited 2022 Jul 6]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-july-2022
3. Korea Disease Control and Prevention Agency. COVID-19 response guideline, edition 9. Cheongju: Korea Disease Control and Prevention Agency; 2020.
4. Shen M, Zhou Y, Ye J, Abdullah Al-Maskri AA, Kang Y, Zeng S, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal 2020;10:97-101.



5. Ryu B, Oh J, Shin M, Kim S, Kim I. Global trends and characteristics of the SARS-CoV-2 Delta variant. Public Health Wkly Rep 2021;14:2354-65.
6. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052-9.



7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42.


8. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;323:1612-4.



9. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763-70.



10. Lee SW, Moon SY, Yon DK. Epidemiological and clinical characteristics of 5,628 patients with coronavirus disease 2019 in South Korea: a nationwide multicenter study. Allergy Asthma Respir Dis 2021;9:136-40.

11. Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health 2020;65:533-46.


12. Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LD, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med 2021;9:139-48.

13. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically ill patients in the Seattle region: case series. N Engl J Med 2020;382:2012-22.


14. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81.



15. Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020;48:e799. -804.


16. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.



17. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9.



18. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62.



19. Fisman DN, Tuite AR. Progressive increase in virulence of novel SARSCoV-2 variants in Ontario, Canada. MedRxiv [Preprint] 2021 Jul 5 .https://doi.org/10.1101/2021.07.05.21260050

20. Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England. London: Public Health England; 2021.
21. Singh C, Naik BN, Pandey S, Biswas B, Pati BK, Verma M, et al. Effectiveness of COVID-19 vaccine in preventing infection and disease severity: a case-control study from an Eastern State of India. Epidemiol Infect 2021;149:e224.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,361 View
- 92 Download
- Related article in KJFM
-
Characteristics of the patients of chronic disease.2000 May;21(5)






