Association between the Urine Cotinine Level and Blood Pressure in Korean Adults with Secondhand Smoke Exposure: Korea National Health and Nutritional Examination Survey 2016–2018
Article information
Abstract
Background
Similar to smoking, exposure to secondhand smoke is a risk factor for developing hypertension and cardiovascular diseases; however, there is no standardized method for measuring smoke exposure. Measuring urine cotinine levels is one possible means for determining the degree of exposure to secondhand smoke. This study investigated the association between urinary cotinine levels and blood pressure in Korean adults exposed to secondhand smoke.
Methods
Data from the Korea National Health and Nutrition Examination Survey conducted between 2016 and 2018 were used. A total of 9,273 participants aged ≥19 years self-reported as current non-smokers, which was cotinine- verified. A complex sample general linear model regression analysis was performed to analyze the association between urine cotinine and blood pressure. A P-value of <0.05 was considered statistically significant.
Results
Corrected urine cotinine levels were positively associated with systolic and diastolic blood pressure in female participants (P<0.001 and P=0.040, respectively). Furthermore, a 10-fold increase in the corrected urine cotinine level of those in contact with secondhand smoke was independently associated with 2.085 mm Hg and 0.575 mm Hg increases in systolic and diastolic blood pressure, respectively. However, there was no association between systolic and diastolic blood pressure in male participants (P=0.226 and P=0.256, respectively).
Conclusion
Urinary cotinine levels were positively associated with increased blood pressure in females exposed to secondhand smoke. Therefore, urinary cotinine may be used as an indicator to quantify and monitor the effects of blood pressure elevation in females exposed to secondhand smoke.
INTRODUCTION
Hypertension is a leading cause of several major human diseases and is a risk factor for developing cardiovascular conditions, such as coronary artery disease, congestive heart failure, and stroke. Over 1 billion people worldwide are affected by hypertension, with over 9.4 million dying from the disease yearly [1]. In Korea, the prevalence of hypertension over the age of 30 years was around 27.2% in 2019, the awareness rate was 71.4%, and the control rate was 48.8% [2]. While the data indicates an improvement over previous years, sufficient recognition and control of high blood pressure (BP) has yet to be achieved.
Smoking is a significant risk factor for hypertension. Similar to smoking, secondhand smoke is a risk factor for developing cardiovascular diseases, including hypertension and several other smoking-related diseases. Research is underway in several countries and efforts are being made to prevent secondhand smoke exposure. In 2019, among non-smokers aged 19 years in Korea, the rates of exposure to secondhand smoke were 4.7%, 14.1%, and 18.3% in homes, indoor working areas, and public areas, respectively [2].
Unlike the “pack-year” unit for quantifying cigarette smoking, there is no standardized method to measure secondhand smoke exposure; moreover, developing a standardized survey to determine the degree of secondhand smoke exposure is challenging. There may be a discrepancy between the perceived degree of exposure and the concentration of smoking constituents inhaled through secondhand smoke [3]. Therefore, research on the effect of secondhand smoke using surveys is limited.
Cigarette components such as nicotine and cotinine play vital roles in BP elevation by increasing peripheral resistance and stimulating sympathetic nerves [4,5]. A recent mouse model study confirmed that chronic nicotine inhalation significantly increases BP [6]. In addition, various studies have been conducted on the effect of smoking on the human body by measuring cotinine levels in various human-derived biological fluids. Although measuring the serum cotinine has been regarded as the traditional method for determining the level of nicotine metabolites, urine and saliva cotinine levels have become universal indicators of smoking status due to their non-invasiveness and suitability for large-scale epidemiological studies [7].
However, to date, few studies have examined whether secondhand smoke negatively affects BP, and previous studies have reported inconsistent results regarding the relationship between secondhand smoke and BP [8,9]. Therefore, we aimed to investigate the relationship between BP and secondhand smoke based on urine cotinine levels in a large cohort database representative of the Korean population.
METHODS
1. Study Population and Design
Data for this study were obtained from the Korea National Health and Nutrition Examination Survey (KNHANES) VII conducted from 2016 to 2018. The KNHANES is an annual national survey conducted by the Korea Disease Control and Prevention Agency (KDCA) to assess the health and nutritional status of the Korean population. In the KNHANES, a professional survey team investigates four regions weekly (192 areas over 48 weeks per year). A three-day survey was conducted in each area, and a mobile examination vehicle visited the sites to conduct a health examination, health interview, and nutrition survey. Since 2007, the KDCA has chosen the Rolling Sampling Survey method to conduct the KNHANES, which was introduced so that independent rolling samples from each year over 3 years (three samples) could be used to represent the whole country.
Among the participants (n=24,269), those aged >19 years with no history of kidney disease and serum creatinine <1.5 mg/dL were selected to reduce the effects of renal function on nicotine metabolism or cotinine concentration (n=16,921). From this group, current self-reported non-smokers, verified by measuring urine cotinine levels, who did not use any cigarettes, e-cigarettes, nicotine replacement products, or other types of cigarettes, were included (n=9,328). Urine cotinineverified non-smokers were defined as those with a urine cotinine-tocreatinine ratio ≤100 μg/g [10]. After excluding participants with missing values, 9,273 participants were included in the final analysis. After calculating the weighted number of Koreans, the total number of participants in the final analysis was 20,663,797 (Figure 1).
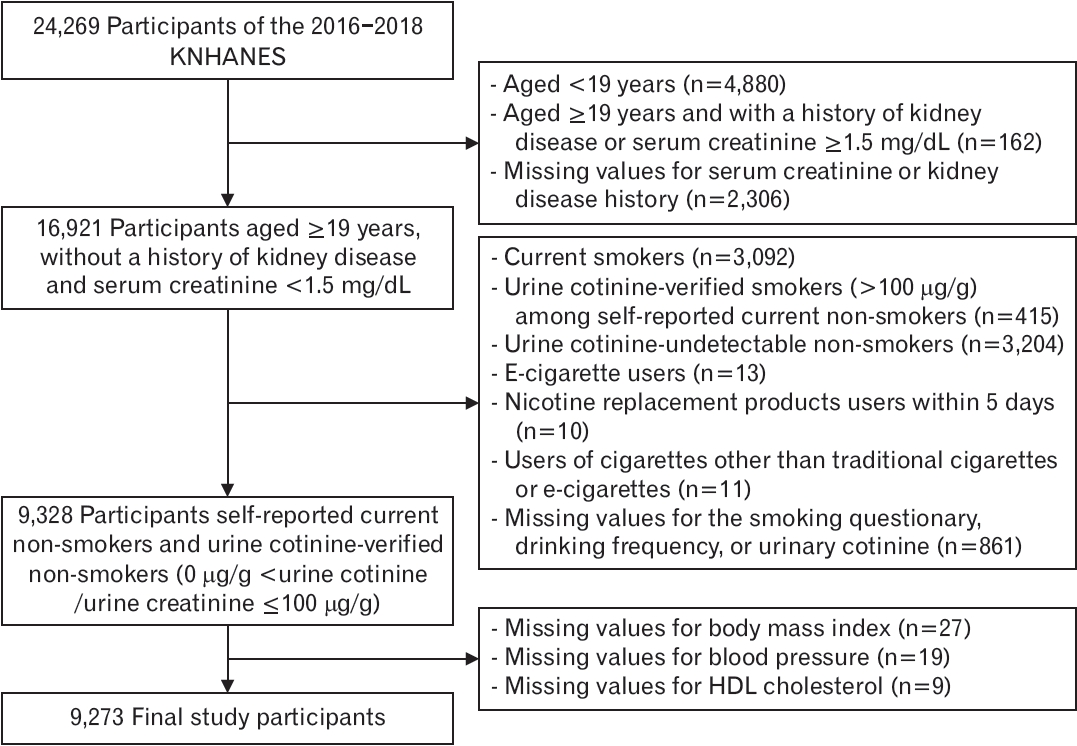
Flow diagram of the study participants. KNHANES, Korea National Health and Nutrition Examination Survey; HDL, highdensity lipoprotein.
Statistical analysis was performed according to sex, considering sex-dependent differences in nicotine metabolism [7], cardiovascular effects in current smokers [11,12], non-smokers [8,13], and the Korean smoking response [3,14].
This study was approved by the local ethics committee of Severance Hospital at Yonsei University (registration no., 4-2022-1181).
2. Secondhand Smoke-exposed Participants
Self-reported current non-smokers were those who indicated on the questionnaire that they smoked in the past, but were not currently smoking or had no history of smoking. To eliminate false reports, those with a urine cotinine-to-creatinine ratio exceeding 100 μg/g were excluded using the study values obtained to distinguish smokers from secondhand smoke-exposed participants in the same study group [10]. The sensitivity analysis secondhand smoke-exposed group consisted of those who answered “yes” to a survey on the presence of secondhand smoke in the workplace, home, or public place.
3. Alcohol Intake
In the 1-year drinking frequency survey, people who answered that they were non-drinkers, did not drink at all in the past year, or drank less than once a month were placed in the ‘<1 time/mo’ group. Those who answered that they drank once a month or 2 to 4 times a month were placed in the ‘1–4 times/mo’ group. Finally, those who answered that they drank 2 to 3 times a week or more than 4 times a week were assigned to the ‘≥2 times/wk’ group.
4. Blood Pressure Measurement and Hypertension
Four nurses from the KDCA professional investigation team conducted the BP and pulse measurements. The BP of adults aged 19 years was measured using a non-mercury automatic BP meter (Watch BP Office AFIB; Microlife, Widnau, Switzerland). When measuring BP, several 4 cm high armrests were used to adjust the arm height to heart level, and the average arm height using the armrests was 83 cm for males and 81 cm for females. Three BP measurements were taken, and the averages of the second and third BP measurements were defined as the absolute systolic blood pressure (SBP) and diastolic blood pressure (DBP). Hypertension was defined as SBP ≥140 mm Hg, DBP ≥90 mm Hg, or the use of hypertension medication.
5. Body Measurement
Weight was measured using a digital scale (InBody 970; BioSpace, Urbandale, IA, USA). Height was measured by using an extensometer (Seca 274; Seca, Hamburg, Germany). The body mass index (BMI) was calculated from the measured values.
6. Blood Tests
Typical blood tests included total cholesterol, triglycerides (TG), highdensity lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, blood urea nitrogen, creatinine, fasting glucose, glycated hemoglobin, hepatitis B surface antigen, hepatitis C antibody, aspartate aminotransferase, alanine aminotransferase, hemoglobin, hematocrit, red blood cell count, white blood cell count, uric acid, and high-sensitivity C-reactive protein (hsCRP).
7. Urine Tests and Urine Cotinine
Urine tests included protein, glucose, urobilinogen, ketones, bilirubin, specific gravity, pH, nitrate, sodium, potassium, microalbumin, cotinine, and creatinine levels. Urine cotinine was measured by HPLC-MS/MS using Agilent 1100 Series with API 4000 (AB Sciex, Framingham, MA, USA). Methylene chloride (Fisher Scientific, Pittsburgh, PA, USA), methanol (Merck, Wiesbaden, Germany), hydrochloric acid (Sigma-Aldrich, Burlington, MA, USA), acetonitrile (Fisher Scientific), and formic acid (Sigma-Aldrich) were used as reagents. The detection limit for urinary cotinine was 0.27399 ng/mL. Values below this level were excluded.
8. Statistical Analysis
All statistical analyses were performed using IBM SPSS ver. 26.0 (IBM Corp., Armonk, NY, USA). Because the KNHANES data were collected using a rolling sampling survey method, the general characteristics of the study participants were analyzed using weighted data (Table 1). P-values were calculated by the weighted Rao-Scott chi-square test for categorical variables, and the weighted t-test for continuous variables was used to assess gender differences. A complex sample general linear model (CSGLM) was used to examine the associations between metabolic parameters, including urine cotinine and BP. In the SPSS software, CSGLM was used for linear regression and variance and covariance analyses of the survey data with complex sampling methods. Statistical significance was defined as a P-value <0.05.
RESULTS
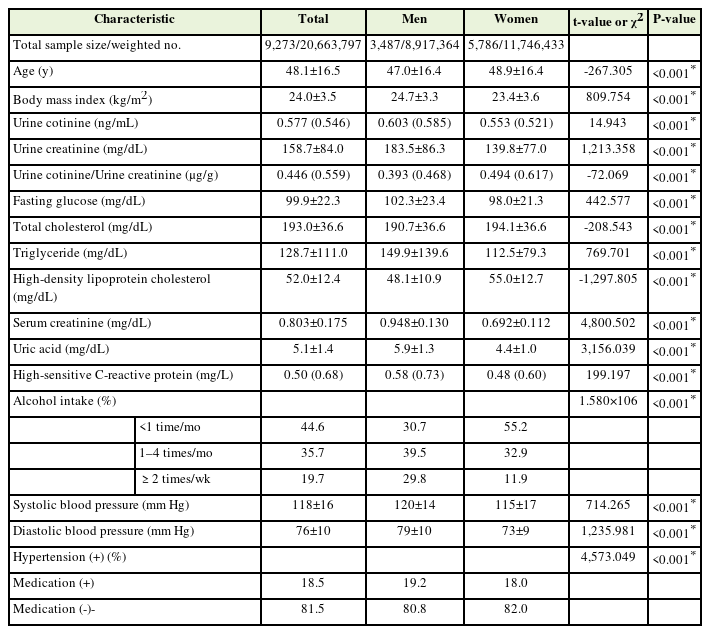
Table 1 presents the general characteristics of the study participants. Of the 9,273 participants (weighted number 20,663,797), 3,487 (weighted number 8,917,364) and 5,786 (weighted number 11,746,433) were males and females, respectively. The mean age for male and female participants was 47.0 years and 48.9 years, respectively. The median urine cotinine level and urine cotinine-to-urine creatinine ratio were 0.603 ng/mL and 0.393 g/g, respectively, in female participants and 0.553 ng/mL and 0.494 g/g, respectively, in male participants. However, the mean BP of male participants (120/79 mm Hg) was higher than that of female participants (115/73 mm Hg).
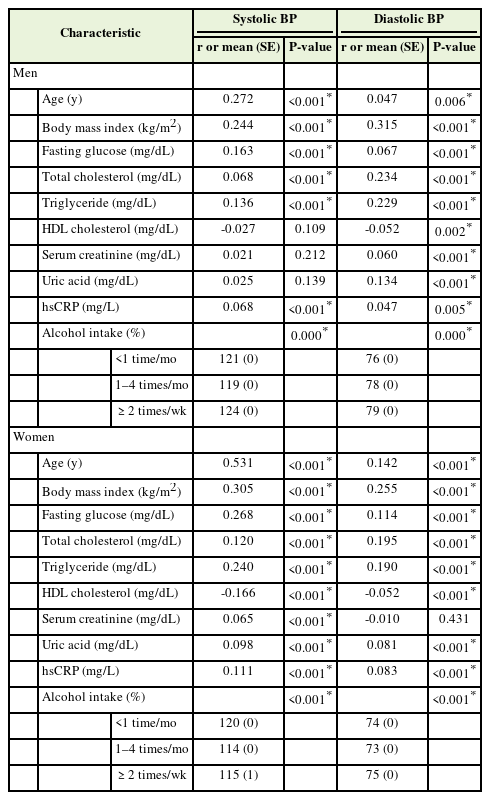
Table 2 shows the correlations between BP and metabolic parameters. Age; BMI; fasting glucose, total cholesterol, TG, and hsCRP levels; and alcohol intake were positively associated with SBP in men. Serum creatinine and uric acid levels positively correlated with DBP, whereas HDL-C negatively correlated with DBP. In females, age; BMI; fasting glucose, total cholesterol, TG, uric acid, and hsCRP levels; and alcohol intake were positively associated with SBP and DBP, whereas HDL-C was negatively associated with SBP and DBP. In females, the serum creatinine levels were positively correlated with SBP.
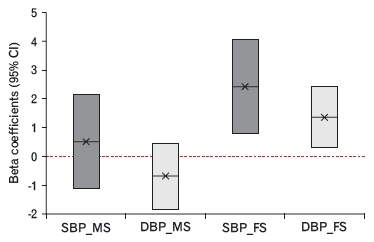
Table 3 and Figure 2 show the independent association between the urine cotinine level and BP. After adjusting for age, BMI, fasting glucose, total cholesterol, TG, HDL-C, uric acid, hsCRP, and alcohol intake, corrected urine cotinine levels were positively associated with SBP and DBP in females (P<0.001 and P=0.040, respectively). Furthermore, a 10-fold increase in the corrected urine cotinine levels in secondhand smoke-exposed subjects was independently associated with increases in SBP and DBP of 2.085 mm Hg and 0.575 mm Hg, respectively. However, there was no association between SBP and DBP in men (P=0.226 and P=0.256, respectively).

Complex general linear model regression analyses to identify an independent relationship between urine cotinine level and BP
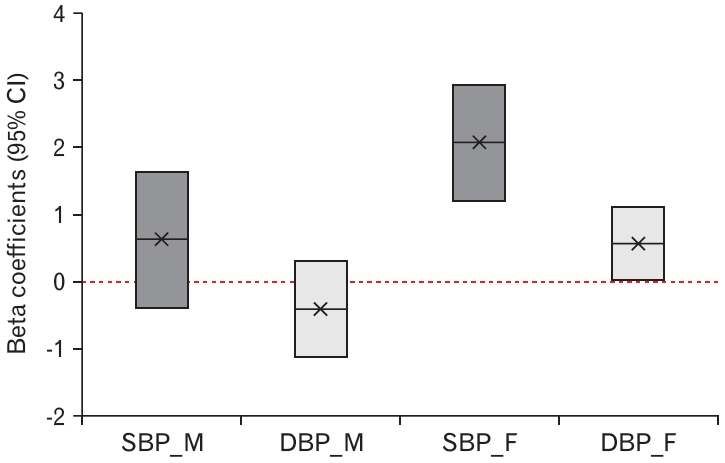
Adjusted association between urine cotinine (μg/g-creatinine) and blood pressure (mm Hg). SBP, systolic blood pressure; DBP, diastolic blood pressure; M, male; F, female; CI, confidence interval.
Appendices 1 and 2 present the results of the sensitivity analyses. As seen in the final study participants, the sensitivity analysis participants exhibited the same pattern of results. After adjusting for BP-related factors, corrected urine cotinine levels were positively associated with SBP and DBP in females (P=0.004 and P=0.012, respectively). Moreover, males demonstrated no association with SBP or DBP (P=0.539 and P=0.233, respectively).
DISCUSSION
In the present study, corrected urine cotinine levels were positively associated with BP in females exposed to secondhand smoke, after adjusting for confounding variables. Similar to first-hand smoking, secondhand smoke is harmful to the lungs, heart, and blood vessels [5,15,16]. Therefore, minimizing secondhand smoke exposure is a global health target, and the early detection and prevention of secondhand smoke exposure are essential. However, there are limitations to solely relying on surveys to determine the effects of secondhand smoke [3].
Nicotine, a component of cigarettes, plays a vital role in elevating BP by increasing peripheral resistance and stimulating sympathetic nerves [4,5]. However, nicotine has a short half-life (approximately 2 hours) and is not a useful quantitative indicator of nicotine intake. Conversely, cotinine, the major proximate metabolite of nicotine, is the most widely used biomarker of nicotine intake; it has a long half-life, and can minimize false positivity [7]. A recent review article indicated that serum cotinine cut-off values for cotinine-verified current smokers ranged from 3.0 to 20 ng/mL, and urine cotinine cut-off values ranged from 31.5 to 550 ng/mL [17]. As such, there is a wide range of urine cotinine cut-off values for classifying smokers and non-smokers. Several studies have shown that corrected urine cotinine levels are a better representation of serum cotinine levels [10,18)].Conversely, in another study, uncorrected values showed a stronger correlation with serum cotinine levels than corrected values [19]. In the present study, corrected urine cotinine levels were used to distinguish non-smokers from smokers. A previous study examined the urine cotinine cutoff values of smokers and nonsmokers based on data from the 7th KNHANES, which comprised the same population as in the present study [10].
Excitation of the sympathetic nerve underlies the effect of nicotine on BP. There are two main mechanisms by which nicotine boosts sympathetic activity: in the first mechanism, it acts directly on the central nervous system to activate the sympathetic nerves. In the central nervous system, specifically in the medulla, the rostral ventrolateral medulla (RVLM) is responsible for the basal and reflex control of sympathetic activity. Nicotine activates the excitatory nicotinic receptors present in the RVLM to excite the sympathetic nerves. The second mechanism involves indirect activation of sympathetic nerves through the inhibition of nitric oxide (NO) production. A lowered NO level suppresses the effect of central sympathetic outflow [5].
Previous studies have evaluated the changes in BP due to smoking or exposure to secondhand smoke. Moreover, studies evaluating the effects of smoking or secondhand smoke exposure on serum or urine cotinine have been conducted since the 1970s and have increased in number since the 2000s.
In a Western study on secondhand smoke, SBP elevation was associated with increased serum cotinine among those in contact with secondhand smok [20], and preschool children with smoking parents had significantly higher SBP and DBP [21]. A recent 2020 study found that among children and adolescents exposed to secondhand smoke, an increase in DBP was observed only in males [13]. In Asia, a Chinese study involving elderly women aged >60 years showed no statistically significant difference in SBP and DBP elevation between those who were exposed to secondhand smoke and those who were not; however, the prevalence of hypertension was significantly higher among the secondhand smoke-exposed participants [22].
A previous study in Korea found that male smokers had considerably lower SBP and DBP levels than those of non-smokers [23]; however, in the case of secondhand smoke, a Korean study observed a significant increase in SBP and DBP among females exposed to secondhand smoke [24]. In addition, another study found that the prevalence of hypertension was higher among secondhand smoke-exposed participants regardless of sex [8]. A recent study also found that the group with reduced secondhand smoke exposure showed a significant decrease in SBP [9].
In the present study, corrected urine cotinine levels were associated with SBP and DBP elevation in females but not in males. Based on the results of this and previous studies, smoking or secondhand smoke tends to cause BP elevation in females rather than males, and affects SBP rather than DBP. Although the exact mechanism is unknown, sex hormones strongly influence cotinine levels, which may partly explain the difference in the relationship between cotinine and BP. The generation of cotinine from nicotine via the liver enzymes CYP2A6 and estrogen induces CYP2A6 and cotinine glucuronidation activity [25]. Moreover, considering that there have been sex-dependent false response rates in smoking questionnaires among Koreans for reasons such as Confucian social norms, a sex analysis should be conducted [3,11,14]. Therefore, future studies are needed to determine the cause of the sex-specific differences in the effects of long-term smoking, secondhand smoke exposure, and nicotine or cotinine accumulation. In addition, as uncorrected urine cotinine has been used in various studies, it is necessary to determine whether corrected or uncorrected urine cotinine better reflects serum cotinine levels in the Korean population.
Liquid e-cigarettes and heat-not-burn cigarettes were introduced in South Korea in 2008 and 2017, respectively. The proportion of heat-not-burn cigarette users compared to liquid e-cigarette users is increasing because of the similarity between heat-not-burn cigarettes and traditional cigarettes. In addition, most people use heat-not-burn cigarettes in their cars and homes [26]. Therefore, the impact of secondhand smoke from heat-not-burn cigarettes has been evaluated in Korea [27,28]. Although the 8th KNHANES, conducted in 2019, investigated liquid e-cigarettes and heat-not-burn cigarettes separately, this was not the case in the 7th KNHANES. Therefore, information on the effects of secondhand smoke is limited. Although the cardiovascular risk of cigarette-type e-cigarettes has been studied abroad [29], there have not been any studies on Koreans; thus, more research is needed in the field.
Our study had some limitations. First, the cross-sectional design of the study does not permit clarification of any cause-and-effect mechanism. Secondly, we could not obtain data on secondhand smoke exposure time, intensity, or frequency, making it difficult to subdivide the characteristics of secondhand smoke exposure. This relationship has not been explored in other populations.
A major strength of this study is the use of a large real-world database representative of the Korean population. In addition, we examined the urinary cotinine levels, which have been used as biomarkers, with and without creatinine correction. These properties render cotinine suitable for large-scale epidemiological studies.
In conclusion, corrected urinary cotinine levels and BP were positively correlated among Korean females exposed to secondhand smoke. Therefore, urinary cotinine may be used as an indicator to quantify and monitor the effect of secondhand smoke exposure on females.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
References
Appendices
Appendix 1. Complex general linear model regression analyses to identify an independent relationship between urine cotinine level and BP among the participants included in the sensitivity analysis
Adjusted associations between urine cotinine (μg/g-creatinine) and blood pressure (mm Hg). SBP_MS, DBP_MS, systolic and diastolic blood pressure among male sensitivity analysis participants; SBP_FS, DBP_FS, systolic and diastolic blood pressure among female sensitivity analysis participants.