 |
 |
- Search
| Korean J Fam Med > Volume 44(4); 2023 > Article |
|
Abstract
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIALS
Supplement┬Ā6.
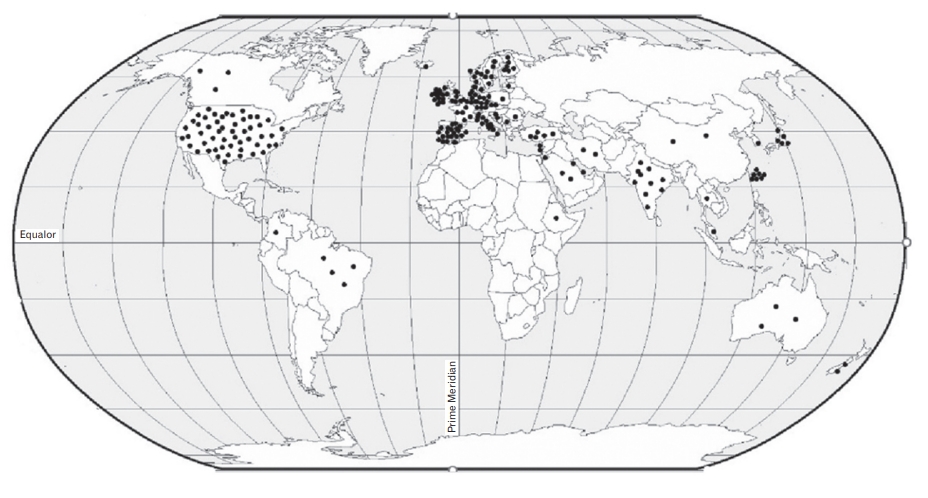
Figure.┬Ā1.

Figure.┬Ā2.
Table┬Ā1.
| Review | Country | No. of included studies | No. of included studies related to PIP | Primary studies from | Aim of SR | Type of included studies | Setting | Population characteristics | Sample size related to PIP | Quality assessment tool | PIP tools/criteria applied |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bhagavathula et al. [27] (2022] (Ethiopia) | Czech Republic | 8 | 7 | Ethiopia | To conduct an SR on the prevalence of polypharmacy and PIM use in older adults in Ethiopia. | Cross-sectional (7) | Inpatient (3), outpatient (1), mixed setting (3) | Population aged Ōēź65 years in Ethiopia | 32 to 1,252 (2,455) | NOS for cross-sectional studies | Beers Criteria 2003, 2012; STOPP; WHO core indicators |
| Bhagavathula et al. [26] (2021)] (India) | Czech Republic | 27 | 27 | India | To perform an SR and meta- analysis to assess the overall prevalence and regional variations of polypharmacy, hyper polypharmacy, and PIM use in older people in India. | Cohort (19), cross-sectional (8) | Outpatient (9), inpatient (15), both (3) | Population aged Ōēź60 years in India | 90 to 1,510 (11,649) | NOS for cross-sectional and cohort studies | Beers Criteria*, 2003, 2012; STOPP/ START; Beers Criteria in combination with STOPP, START, MAI, PhadkeŌĆÖs criteria |
| Bories et al. [12] (2021) | France | 91 | 91 | Global: Asia (10), Europe (50), North America (22), South America (7), Oceania (2) | To perform an SR in hospital, nursing home, and primary care settings to evaluate the prevalence and severity of DDI in older patients for whom PIMs are prescribed, and to evaluate the DDI specifically involving PIM. | Prospective (29), retrospective (62) | Nursing home (20), primary care (38), hospital (33) | Population aged Ōēź60 yearsŌĆĀ | 24 to 1,549,824 (8,082,985) | Not mentioned | Beers Criteria 1991, 1997, 2003, 2012, and 2015; STOPP/START; STOPP; START; PRISCUS; SNBHW; Fick criteria; NORGEP; Zhan criteria; McLeod criteria; MAI; IPET; WHO criteria; Swedish National Formulary; Laroche; Oncostrip; EU (7) PIM list; Winit-Watjana; Mimica Matanovic and Vlahovic-Paleevski; AMDA Guideline; VHA/DOD Guideline; Topinkova list; expert panel; drug-disease combinations; NICE; British National Formulary; SIGN; combination of these tools |
| Chang et al. [28] (2021) | Malaysia | 27 | 11 | Malaysia | To estimate the pooled prevalence of drug- related problems and complementary and alternative medicine use among the older Malaysian population. | Cross-sectional (9), cohort (1), RCT (1) | Nursing homes (5), hospitals (3), clinics (2), community (1) | Population >60 years old in Malaysia | 90 to 1,256 (3,784) | JBI | Beers Criteria*; STOPP/START; STOPP; MAI |
| Malakouti et al. [29] (2021) | Iran | 13 | 13 | Global: Asia (3), Europe (7), North America (2), Oceania (1) | To review PIMs and their related costs among older adults internationally. | Cross-sectional (8), cohort (3), retrospective (2) | Residential care home (2), hospitals (1), outpatient (2), community (8) | Population aged Ōēź60 yearsŌĆĪ | 236 to 4,475,067 (6,285,034) | JBI | Beers Criteria*, 2003, 2015; Japanese modified Beers Criteria; STOPP; PRISCUS; EU (7) PIM list; European PIMs list |
| Ude-Okeleke et al.31) (2021) | UK | 21 | 9 | Global: Europe (6), North America (2), Oceania (1) | To explore published primary studies on MRPs occurring in older people from primary health care settings, including those resulting in hospitalization and to explore the types of tools/interventions employed to identify and prevent MRPs in this patient group. | Cohort study (3), RCT (1), case control study (1), secondary data analysis (3), retrospective study (1) | Nursing home (1), hospital (4), community (3), mixed setting (1) | Population aged Ōēź65 years | Not reported | A modified version of Down and Black tool | Beers Criteria*; PRISCUS; STOPP; STOPP/START; Australian criteria for assessment of prevalence |
| Xu et al. [35] (2021) | China | 50 | 50 | Global: Asia (12), Europe (26), North America (7), Oceania (5) | To identify the factors associated with PIPs among older adults in primary care settings and to identify from stake-holders the perceived implementation barriers to the optimization of medicines and their recommended potential solutions. | Cohort (2), cross- sectional (34), qualitative studies (14) | Primary care (50) | Population aged Ōēź65 years | 89 to 1,595,054 | JBI | Beers Criteria 1991, 1997, 2002, 2003, 2012, 2015, and 2019; STOPP/START; STOPP; PRISCUS; Fick criteria; NORGEP; Zhan criteria; McLeod criteria; Winit-Watjana; MAI; FORTA list; GheOP3S tool; Maio criteria; HEDIS HRME |
| Liew et al. [11] (2020) | Singapore | 66 | 66 | Global: Asia (7), Europe (46), North America (9), South America (2), Oceania (2) | To conduct an SR with meta-analysis to estimate the overall prevalence of PIP among older persons in primary care, to determine the potential impact of PIP in primary care, and to estimate the proportion of adverse effects related to older persons in primary care that can be explained by PIP | Cohort (12), cross- sectional (50), baseline data of RCT (4) | Primary care (66) | Population aged Ōēź65 years┬¦ | 50 to 1,360,251 (5,054,975) | NOS modified for prevalence studies | Beers Criteria 1997, 2003, 2012, 2015; STOPP/START; STOPP; START; PRISCUS; Maio criteria; MAI; HEDIS; Zhan criteria; DUR; IPET; ACOVE PIM; ACOVE PPO; FORTA; Winit-Watjana; HMRE; expert panel; combination of these tools |
| Weeda et al. [34] (2020) | USA | 21 | 21 | Global: Asia (8), Europe (6), North America (6), Oceania (1) | To conduct a meta-analysis to evaluate the impact of PIM use compared to no PIM on hospital encounters. | Cohort (19), cross- sectional (1), case- crossover (1) | Not mentionedŌłź | Older adultsŌĆÖ patientsŌĆĪ | 184 to 1,807,404 | GRACE | Beers Criteria 1997, 2002, 2003, 2012; STOPP; PRISCUS; SNBHW; HEDIS; NCQA; DDI; DD; Japanese Guideline for Medical Treatment and Its Safety in Elderly; Narrow Therapeutic Index Drugs; combination of these tools |
| Nothelle et al. [33] (2019) | USA | 22 | 22 | USA | To systematically review literature to identify the determinants of PIM use in older community-dwelling adults in USA. | Cross-sectional (17), cohort (4), survey (1) | Community-dwelling and ambulatory clinic patients (22) | Population aged Ōēź65 years in USA┬Č | Not reported | Critical Appraisal Checklist (Center for Evidence-Based Medicine) | Beers Criteria 1997, 2002, 2003, 2012, 2015; Zahn criteria; STOPP; HEDIS; DDI; modified Fick criteria; therapeutic duplication; Beers Criteria 2012/anticholinergics only; Beers Criteria 2012/2015/sedatives or antidepressants; Beers Criteria 2012/drugs inappropriate in diabetes; Beers Criteria 2003/inappropriate in cognitive impairment; benzodiazepine receptor agonists and nonbenzodiazepine sleep aids; Elsevier Gold Standard and VA provider input; combination of these tools |
| Thomas and Thomas [30] (2019) | Canada | 62 | 62 | Global: Asia (15), Africa (3), Europe (26), North America (11), South America (5), Oceania (2) | To identify all the studies using the updated 2015 STOPP/START and American Geriatric Society 2015 Beers Criteria and assess whether studies with lower rates of inappropriate prescribing had lower rates of emergency and hospital visits, medication adverse effects, and mortality. | RCT (2), non-randomized (databases, surveys, and retrospective and prospective chart reviews) (60) | Community (32), hospital (30) | Population aged Ōēź65 years┬¦ | 51 to 1,122,080 (1,854,698) | 1) Cochrane Collaboration Risk of Bias Tool kit for RCT studies | STOPP/START 2015; Beers Criteria 2015; STOPP/START 2015 and Beers Criteria 2015 |
| 2) STROBE for nonrandomized studies | |||||||||||
| Muhlack et al. [32] (2016) | Germany | 16 | 16 | Global: Asia (2), Europe (7), North America (6), Oceania (1) | To identify, appraise, and meta-analyze the available evidence on the association of PIM intake with mortality and cardiovascular events from cohort studies. | Cohort study (16) | Community-dwelling (9), inpatients (2), outpatients (1), nursing home (2), mixed setting (2) | Population >60 years of age and not restricted to having one specific disease# | 425 to 1,807,404 | NOS for assessing the quality of nonrandomized studies | Beers Criteria 1991, 1997, 2003, 2012; HEDIS-DAE; Maio criteria; SNBHW; Beers Criteria along with McLeod criteria, Elliot criteria, DUR, Duplications and Interactions |
| Thomas [14] (2016) | Canada | 29 | 29 | Global: Asia (3), Europe (25), Oceania (1) | To identify all the related studies in all languages and summarize the following rates in individuals Ōēź65 years (i) PIMs identified using STOPP criteria and (ii) PPOs identified using START criteria, in the literature to date and to (iii) update the 2011 review by Hill-Taylor | Not mentioned | Nursing home (6), community (5), clinic (5), hospital (13) | Population aged Ōēź65 years | 50 to 1,019,491 (1,537,197) | Not mentioned | STOPP and START criteria |
| Tommelein et al. [5] (2015) | Belgium | 52 | 52 | Europe | To synthesize observational research on the prevalence of PIP in community- dwelling older adults in Europe, to present an overview of the risk factors mostly described in association with PIP, and to summarize the drugs or drug groups most often involved in PIP. | Observational studies (52) | Community-dwelling (52) | Population aged Ōēź65 year in Europe | 50 to 1,019,491 | Slightly adapted quality assessment scale from the Cochrane Collaboration group | Beers Criteria 1991, 1997, 2003, 2012; STOPP; START; PRISCUS; NORGEP criteria; MAI; McLeod criteria; Matanovic criteria 2012; SQIs; ABCD categorization; Beers Criteria in compilation or along with DDIs, Laroche, PRISCUS, IPET, STOPP/START, McLeod criteria; adaptations of criteria |
| Elmst├źhl and Linder [25] (2013) | Sweden | 24 | 10 | Global: Europe (6), North America (3), Oceania (1) | To determine the prevalence of polypharmacy in the general older adult population Ōēź65 years, and to determine the prevalence of inappropriate drug use in the same group. | Crosssectional (5), cohort (2), retrospective (2), prospective (1) | Population (4), hospital (2), emergency care (1), primary care (1), nursing home (1), mix setting (1) | Population Ōēź65 years | 543 to 626,258 | Not reported | Beers Criteria 1997, 2003; McLeod criteria; Zahn criteria; SNBHW |
| Hill-Taylor et al. [23] (2013) | Ireland | 13 | 13 | Global: Asia (1), Europe (11), North America (1) | To describe the application of STOPP/ START criteria and to examine the evidence of the impact of STOPP/START on clinical, humanistic, and economic outcomes in older adults. | Observational studies (12), RCT (1) | Community dwelling (2), hospital (7), mixed setting (1), long-term care (3) | Population aged Ōēź65 yearsŌĆĪ | 100 to 338,801 (344,957) | QUIPS | STOPP/START; Beers Criteria (2002); MAI; AOU; Australian criteria |
| Opondo et al. [22] (2012) | Netherlands | 19 | 19 | Global: Asia (4), Europe (8), North America (7) | To identify and summarize published studies on IMP in older adults in primary care, to quantify its extent, and to identify medications for which interventions may be implemented to improve medication prescription quality. | Not mentioned | Outpatient clinics, office practice, general practice, and primary health care clinics (19) | Population aged Ōēź65 years | 100 to 12,513,584 | Not mentioned | Beers Criteria 1991, 1997, 2003; modified Beers 2003; Zhan criteria; Beers/Zhan; HEDIS HRME; MRPS list |
| Guaraldo et al. [24] (2011) | Brazil | 19 | 19 | Global: Asia (1), Europe (1), North America (16), Oceania (1) | To identify and describe studies that used information from insurance company and social security administrative databases to assess IMU among community-dwelling older adults and to present the risk factors most often associated with IMU. | Not mentioned | Community-dwelling (19) | Community-dwellers aged Ōēź60 years | 777 to 2,133,864 | Some items from STROBE were used for quality assessment | Beers Criteria 1991, 1997, 2002; Beers Criteria 2002; McLeod; Beers Criteria 1991/1997; adaptations of Beers Criteria; HEDIS; Zhan criteria |
| Liu and Christensen [13] (2002) | USA | 11 | 11 | USA | To provide an updated review of the recent empirical studies on inappropriate prescription drug use among older adults using the 1997 Beers Criteria. | Cohort (1), crosssectional (10) | Nursing home (1), community (6), ambulatory clinic (1), hospital (1), home health care (1), homeless older adults (1) | Elderly patients in USA | 146 to 64,832 | Not mentioned | Only Beers Criteria 1997 |
PIP, potentially inappropriate prescribing; SR, systematic review; PIM, potentially inappropriate medication; NOS, Newcastle Ottawa Scale; STOPP, Screening Tool of Older PersonsŌĆÖ Prescriptions; START, Screening Tool to Alert to Right Treatment; DDI, drugŌĆōdrug interactions; RCT, randomized controlled trial; JBI, Joanna Briggs Institute critical appraisal checklist; MRPs, medicines related problems; PPO, potential prescribing omission; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; IMP, inappropriate medication prescription; IMU, inappropriate medication use; WHO, World Health Organization; MAI, Medication Appropriateness Index; PRISCUS, Latin for ŌĆ£old and venerableŌĆØ; SNBHW, Swedish National Board of Health and Welfare Quality indicator; NORGEP, The Norwegian General Practice; IPET, Improving Prescribing in the Elderly Tool; EU, The European Union; AMDA, American Medical Directors Association; VHA/DOD, The Veterans Health Administration and Department of Defense; NICE, The National Institute of Clinical Excellence; SIGN, Scottish Intercollegiate Guidelines Network; FORTA, Fit For The Aged list; (GheOP3S)-tool, The Ghent Older People's Prescriptions community Pharmacy Screening; HEDIS, Healthcare Effectiveness Data and Information Set; HRME, High-Risk Medications in the Elderly; DUR, Drug Utilization Review; ACOVE PIM, Assessing Care of Vulnerable Elders Potentially Inappropriate Medications; ACOVE, Assessing Care of Vulnerable Elders; GRACE, The Good ReseArch for Comparative Effectiveness; NCQA, National Committee for Quality Assurance; HEDIS-DAE, Healthcare Effectiveness Data and Information Set-Use of High-Risk Medications in the Elderly; SQI, Swedish Quality Indicators; QUIPS, Quality in Prognostic Studies; AOU, Assessment of Underutilization index.
ŌĆĀ Among 91 studies, 25 were conducted on specific populations (populations with specific medications or diseases, such as oncology and psychiatric diseases).
┬¦ Five studies were conducted on specific populations (those with dementia, cancer, hypertension, generalized anxiety disorder, depressive symptoms, and atrial fibrillation patients).
Table┬Ā2.
| Review | No. of primary studies conducted in community settings | No. of excluded primary studies by reason |
The primary studies that met our eligibility criteria to report the prevalence of PIP |
Overall prevalence of PIP (range) | Meta-analysis related to the prevalence of PIP | Pooled prevalence of PIP in community settings (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| No. | Sample size | PIP tools/criteria applied | ||||||
| Bhagavathula et al. [27] (2021) (Ethiopia) | 1 | 0 | 1 | 392 | Beers Criteria (1) | 6.3% | Yes; The meta-analysis was not conducted by different settings | - |
| Bhagavathula et al. [26] (2021) (India) | 9 | 0 | 9 | 4,089 | Beers Criteria (7), Beers/other criteria* (2) | 10.8%ŌĆō41.9% | Yes | 25% (19%ŌĆō31%) |
| Bories et al. [12] (2021) | 38 | 11: Specific pathologies (8). the prevalence of PIP not reported (1), mixed settings (1), non-community setting (1) | 27 | 7,692,396 | Beers Criteria (10), SNBHW (5), Beers/other criteriaŌĆĀ (3), MAI (2), Fick (1), McLeod (1), PRISCUS (1), EU 7 PIM list (1), Zhan/Fick (1), Mimica Matanovic and Vlahovic-Paleevski (1), expert panel (1) | 2.58%ŌĆō93.9%ŌĆĪ | No; There were some missing data precluding a formal meta-analysis and its statistical evaluation. | - |
| Chang et al. [28] (2021) | 3 | 2: A specific group of medications (1), the prevalence of PIP not reported (1) | 1 | 1,256 | Beers Criteria (1) | 31.8% | Yes; The calculation of I2 and P-value was not applicable because the pooled effect size was derived from one individual study | 31.8 % (29.3%ŌĆō34.4%) |
| Malakouti et al. [29] (2021) | 10 | 0 | 10 | 6,282,896 | Beers Criteria (5), Beers/other criteria┬¦ (1), STOPP (2), Fick (1), PRISCUS (1) | 8.6%ŌĆō57.2% | No; Meta-analysis could not be performed because of the heterogeneity across studies | - |
| Ude-Okeleke et al. [31] (2021) | 3 | 1: The prevalence of PIP not reported (1) | 2 | Not reported | STOPP (1), Australian criteria (1) | 42%ŌĆō48.7% | No; Meta-analysis could not be performed because of the heterogeneity of the included studies. | - |
| Xu et al. [35] (2021) | 50 | 14: Qualitative studies (14) | 36 | 7,782,784 | Beers Criteria (11), Beers/other criteriaŌłź (9) | 10.4%ŌĆō97.1% | No; Meta-analysis was considered inappropriate due to sample heterogeneity, measurement, and analysis methods. | - |
| STOPP/START (6), STOPP (4), NORGEP (1), Zhan/Fick (1), GheOP3S tool (2), Maio criteria (1), HEDIS HRME (1) | ||||||||
| Liew et al. [11] (2020) | 66 | 5: Specific pathologies (7) | 61 | 5,046,168 | Beers Criteria (19), Beers/other criteria┬Č (17), STOPP/START (12), STOPP (6), PRISCUS (2), MAI (1), START (1), Maio criteria (1), HEDIS HMRE (1), expert panel (1) | 0%ŌĆō77% | Yes; The pooled effect size was 33.3, but this included 5 studies conducted on specific pathologies | - |
| Weeda et al. [34] (2020) | 16 | 4: Mixed settings (3), specific pathology (1) | 12 | 2,533,738 | Beers Criteria (6), STOPP (2), PRISCUS (2), HEDIS (1), Beers/NCQA (1) | 3.5%ŌĆō88% | Yes; Meta-analysis on the prevalence of PIP was not conducted | - |
| Nothelle et al. [33] (2019) | 22 | 12: Specific pathologies (8), a specific group of medications (3), the prevalence of PIP not reported (1) | 10 | Not reported | Beers Criteria (7), Zhan (1), HEDIS (1), Zahn/Fick/Therapeutic duplication/DDIs (1) | 4%ŌĆō51% | No; The results were not amenable to quantitative pooling given the heterogeneity in design across studies. | - |
| Thomas and Thomas [30] (2019) | 32 | 10: RCT (1), a specific group of medications (4), specific pathologies (5) | 22 | 645,619 | STOPP/START (13), Beers Criteria (4), STOPP/START and Beers Criteria (5) | 8%ŌĆō91% | No; The reason was not mentioned. | - |
| Muhlack et al. [32] (2016) | 9 | 2: The prevalence of PIP not reported (1), specific pathologies (1) | 7 | 1,947,012 | Beers Criteria (5), HEDIS (1), Beers/Elliot/McLeod (1) | 5.2%ŌĆō48.7% | Yes; Meta-analysis on the prevalence of PIP was not conducted | - |
| Thomas [14] (2016) | 10 | 0 | 10 | 1,530,937 | STOPP (5), STOPP/START (5) | 14.6%ŌĆō69% | No; Because of heterogeneity in populations and in how the criteria were assessed, the author did not perform a meta-analysis and instead used computed weighted averages to summarize PIP in the included studies. | - |
| (PIM: 30.8%#) | ||||||||
| (PPO: 27.5%#) | ||||||||
| Tommelein et al. [5] (2015) | 52 | 0 | 52 | 8,394,612 | Beers Criteria (18), Beers/other criteria** (13), STOPP/START (6), PRISCUS (4), STOPP (3), MAI (2), Laroche (2), Matanovic (1), SQIs (1), NORGEP (1), ABCD categorization (1) | (0%ŌĆō98%) | No; Due to the heterogeneity of the methodologies applied in the included studies, the results were merely reported in a descriptive way. | - |
| 22.6%# | ||||||||
| Elmst├źhl and Linder [25] (2013) | 5 | 1: Mixed setting (1) | 4 | 847,733 | Beers/other criteriaŌĆĀŌĆĀ (2), Zhan (1), SNBHW (1) | 13%ŌĆō55% | No; Meta-analysis was not used because of the heterogeneity of study design and care settings, as well as the high variation in drop-out rates | - |
| Hill-Taylor et al. [23] (2013) | 2 | 0 | 2 | 340,130 | Full STOPP/START (1), partial STOPP (1) | 21.4%ŌĆō36% | No; Heterogeneity of study populations, interventions and study design precluded meta-analysis. | - |
| Opondo et al. [22] (2012) | 19 | 0 | 19 | 19,895,551 | Beers Criteria (15), Beers/Zhan (1), Zhan (1), HEDIS HRME (1), MRPS list (1) | 2.9%ŌĆō38.5% | No; The reason was not mentioned | - |
| Overall median rate: 20% | ||||||||
| Guaraldo et al. [24] (2011) | 19 | 0 | 19 | 8,965,149 | Beers Criteria (12), Zahn (4), Beers/other criteriaŌĆĀŌĆĀ (2), HEDIS (1) | 11.5%ŌĆō62.5% | No; The reason was not mentioned | - |
| Liu and Christensen [13] (2002) | 8 | 1: A specific group of medication (1) | 7 | 90,609 | Beers Criteria (7) | 14.3%ŌĆō28% | No; The reason was not mentioned | - |
PIP, potentially inappropriate prescribing; CI, confidence interval; SNBHW, Swedish National Board of Health and Welfare Quality indicator; MAI, Medication Appropriateness Index; PRISCUS, Latin for ŌĆ£old and venerableŌĆØ; EU, The European Union; PIM, potentially inappropriate medication; STOPP, Screening Tool of Older PersonsŌĆÖ Prescriptions; START, Screening Tool to Alert to Right Treatment; NORGEP, The Norwegian General Practice; (GheOP3S)-tool, The Ghent Older PeopleŌĆÖs Prescriptions community Pharmacy Screening; HEDIS, Healthcare Effectiveness Data and Information Set; HRME, High-Risk Medications in the Elderly; NCQA, National Committee for Quality Assurance; DDI, drugŌĆōdrug interactions; RCT, randomized controlled trial; PPO, potential prescribing omission; SQI, Swedish Quality Indicators; MRPs, medicines related problems.
ŌĆĪ The overall estimated weighted prevalence of PIP in 30 primary studies was reported to be 19.1%.
Ōłź STOPP/START (4), STOPP (3), McLeod (1), Winit-Watjana (1), MAI (1), FORTA list (1), and Zhan (1). ┬ČSTOPP/START (6), STOPP (6), PRISCUS (1), MAI (1), Zhan criteria (2), DUR (1), IPET (1), ACOVE PIM (1), ACOVE PPO (1), FORTA (1), and Winit-Watjana (1).
REFERENCES
- TOOLS







