Global Mapping of Potentially Inappropriate Prescribing for Older Adults in Community Settings: An Umbrella Review
Article information
Abstract
Potentially inappropriate prescribing (PIP) is a major public health concern with several undesirable health consequences for older adults. In this overview, we aimed to map and gather information from existing literature to provide a better insight into the prevalence of PIP among community dwellers. Electronic databases were searched from their inception to April 2022. The quality of the included systematic reviews (SRs) was assessed using the assessment of multiple systematic reviews checklist. The degree of overlap within the SRs was also evaluated (2% overlap). All SRs on the prevalence of PIP in older individuals in community settings were included, and a narrative approach was used to synthesize data. Nineteen SRs comprising 548 primary studies met the inclusion criteria, and the average quality of the included SRs was moderate. More than half (50.5%) of the primary studies were conducted in Europe, followed by the United States (22.8%), and Asia (18.9%). Thirty different criteria were used in the primary studies to estimate the prevalence of PIP. The most widely used criteria were those presented in Beers (41.8%) and STOPP (Screening Tool of Older Persons’ Prescriptions)/START (Screening Tool to Alert to Right Treatment) (21.8%) criteria. Benzodiazepines, nonsteroidal anti-inflammatory drugs, and antidepressants were the most frequently reported PIPs. A considerable variation in the prevalence of PIP ranging from 0% to 98% was reported by SRs. However, there is a high degree of uncertainty regarding the extent of PIP in community settings. To identify knowledge-to-action gaps, SR authors should consider the differences in prevalence of PIP according to settings, applied tools, data sources, geographical areas, and specific pathologies. There is also a need for primary and SR studies from low- and middle-income countries regarding the prevalence of PIP.
INTRODUCTION
Older adults often experience multiple chronic conditions due to agerelated changes. Approximately 55%–98% of the general older population are considered to have multimorbidity [1], which means that they use a high number of medications. Therefore, compared to other age groups, they are more prone to drug-related problems, including potentially inappropriate prescribing (PIP) [2].
PIP includes prescribing medications that have fewer benefits compared to their harmful effects and/or medications that have not been prescribed, despite the existence of sufficient evidence that the patient benefits from them [3]. According to this definition, PIP includes potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs). The term PIM refers to medications that have a greater risk than benefit, and when used by older adults, they may outweigh the clinical advantages of the drug. However, PPO occurs when a patient does not receive medications with potential benefits for his/her treatment and/or prevention of a condition [3,4]. PIP is associated with increased healthcare expenditure [5]. There are some links between PIP and adverse drug events (ADEs) in older adults, which can lead to avoidable ADEs such as falls [3], fractures, and delirium [6]. Previous studies have also identified associations between PIP and hospitalization [7,8] and mortality. [9].
As an important public health concern, PIP is highly prevalent in different healthcare settings [10]. A recent systematic review (SR) reported the prevalence of PIP among older adults in primary care settings to be approximately 33% [11]. Although most previous studies have estimated the prevalence of PIP to be high, different ranges of prevalence have been observed. Part of this discrepancy is related to different study settings [12,13]; however, even in the same setting, the prevalence of PIP has been reported to vary widely. An SR conducted in Europe showed that the prevalence ranged from 0% to 98% among community-dwelling older adults [5]. Another SR, conducted on the primary studies in community settings, reported a prevalence of 14%–69% [14]. Therefore, despite several studies, evidence regarding the extent of PIP in community setting remains uncertain.
Without mapping the current literature in terms of prevalence of PIP, effective future healthcare planning for updating advice and preventive actions to reduce PIP are challenging. With the increase in the number of SRs, an appropriate next step is to conduct reviews of existing SRs. This allows for the comparison of separate results of SRs and thereby provides healthcare policy makers with sufficient evidence. An umbrella review or overview of SRs is a relatively new approach that summarizes evidence from multiple research syntheses [15,16]. Moreover, as the majority of older adults live in communities, it is important to investigate the prevalence of inappropriate medication use among them to inform and increase the value of future research. Thus, this umbrella review aimed to synthesize the available SRs related to the prevalence of PIP in community-dwelling older adults (aged ≥60 years) and provide evidence-based recommendations for improving future research, both for primary studies and SRs.
MATERIALS AND METHODS
1. Protocol and Registration
Based on the definitions of prevalence and incidence reviews, the study question for this overview was developed in accordance with the CoCoPop framework, which refers to condition, context, and population [17]. The present overview was performed in accordance with the Preferred Reporting Items for the Overview of Reviews statement (Supplement 1). The protocol for this overview is registered in the International Prospective Register of Systematic Reviews (registration no., CRD42021278126). The ethics committee of Tabriz University of Medical Sciences approved the study (IR.TBZMED.REC.1401.208).
2. Search Strategy
Medline (via PubMed), EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Scopus, and Google Scholar were searched from inception to April 29, 2022, without publication time restrictions. The databases were searched using a combination of Medical Subject Headings (MeSH) and free-text terms. Boolean operators AND/OR were applied to combine the search terms. The search strategies are presented in Supplement 2. A manual search of the reference lists of retrieved studies was also conducted to obtain further relevant studies (“snowballing” strategy). Furthermore, the search was supplemented by checking with specialized registers and experts in the field of study. In the present study, we did not explore grey literature because we preferred to limit SRs to those that underwent a peer review process. A trained research librarian assisted the research team with the search process.
3. Inclusion and Exclusion Criteria
All SRs and/or meta-analyses in which the prevalence of PIP in older people (≥60 years) in community settings was reported were included in the overview. Other types of reviews, including narrative and scoping reviews, original studies, theses, and overviews, were excluded. The SRs conducted in any community settings were included. Outpatient, ambulatory, and specialist clinics were defined as community settings because these centers provide services to people living within communities [18]. We also included SRs conducted in primary care settings. SRs conducted in hospitals, emergency departments, long-term care centers, and nursing homes were excluded. SRs restricted to a specific group of medications (such as benzodiazepines, antipsychotics, and anticholinergics) or specific pathologies (such as dementia, cardiovascular diseases, cancer, and frailty) were excluded. We also excluded SRs that did not specifically focus on older age groups. Only SRs published in English were included.
To report the prevalence of PIP solely in a community setting, the research team reviewed the primary studies included in the SRs. In the SRs performed on primary studies from different settings, the prevalence of PIP was reported only in primary studies conducted in community settings. Therefore, primary studies conducted in other mixed settings (such as inpatient and outpatient or a combination of community and other settings) were excluded.
4. Study Selection and Data Extraction
Search results were exported to Endnote X9 software (Clarivate, Philadelphia, PA, USA), and duplicate publications were excluded. Two authors (N.G.A. and F.S.) independently selected SRs based on the eligibility criteria and extracted the data. The titles and abstracts of the selected papers were screened. Then, the full texts of potentially eligible SRs were evaluated according to the inclusion and exclusion criteria. Any disagreements or uncertainties were resolved through discussion with a third author (H.N.).
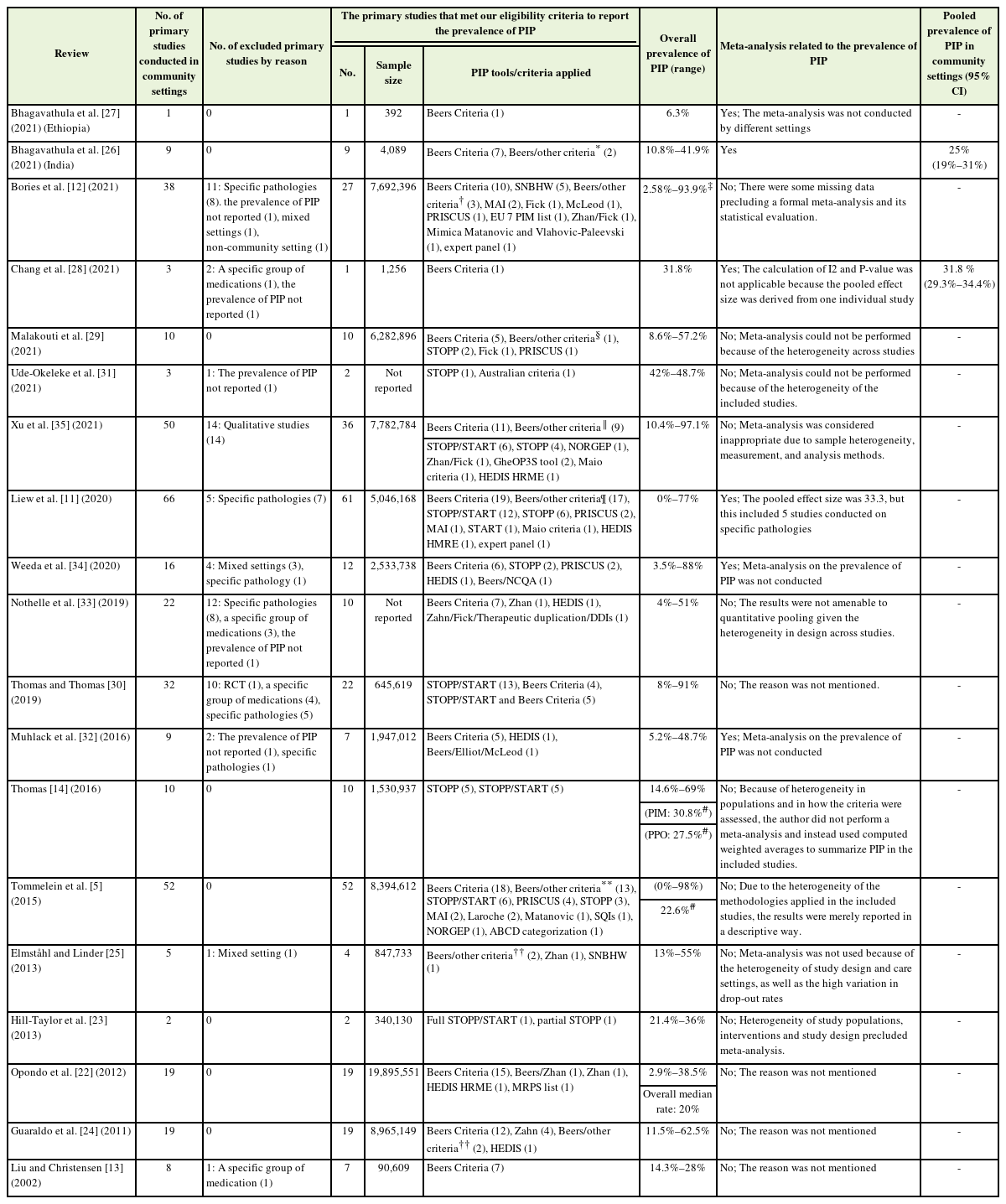
Data were extracted from the included SRs using a data extraction form that included the following information: last name of the first author, publication year and country, number of included studies, number of included studies related to PIP, aim of the study, type of included studies, setting, population characteristics and their total number, applied quality assessment tools, and applied PIP criteria. The following information was extracted from the primary studies: the number of primary studies conducted in community settings, the number of primary studies excluded by reason, the number and sample size of primary studies conducted in community settings, prevalence of PIP, meta-analyses related to prevalence of PIP, and pooled prevalence of PIP.
The extraction forms were pilot-tested on five included SRs and revised accordingly. We attempted to contact the SR authors to obtain further information when the collected data were ambiguous or incomplete. When the authors did not reply, reminder e-mails were sent.
5. Quality and Overlap Assessment
The methodological quality of the included SRs was assessed using the Assessment of Multiple Systematic Reviews (AMSTAR) checklist. The AMSTAR is a validated 11-item tool used to evaluate the quality of SRs. This widely used tool has a significant inter-rater reliability [19]. Compared with other quality assessment tools, AMSTAR is reported to be completed more quickly, with better agreement between the inter-raters [20].
Each question in AMSTAR has four possible responses: “yes,” “no,” “cannot answer,” and “not applicable. [16]" The answer “yes” receives a score of 1, and the other answers receives a score of 0. Therefore, the total quality score ranges from 0 to 11. The quality of the SRs was classified into three levels: scores 8–11 as high quality, scores 4–7 as moderate quality, and scores 0–3 as low quality [18].
The quality of evidence in the primary studies was evaluated according to the assessments reported by the SR authors. We assessed all SRs using a quality assessment tool and reported the author’s judgment regarding the quality of the primary study. The quality of the SRs was independently evaluated by two authors (N.G.A. and F.S.). Any disagreements were resolved with the cooperation of the third author (H.N.). Quality ratings were used in the synthesis process and the results were interpreted.
To measure the degree of overlap within the SRs, the corrected covered area (CCA) recommended by Pieper et al. [21] was applied. The CCA is a value between 0% and 100%. According to Pieper et al. [21], a CCA of 0%–5% is regarded as slight overlap, 6%–10% as moderate overlap, 11%–15% as high overlap, and values >15% are considered a very high overlap.
6. Data Synthesis
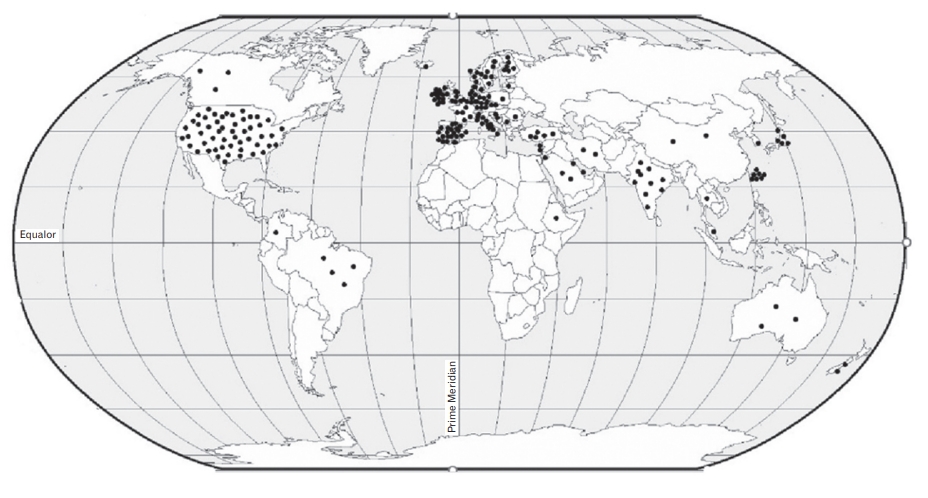
Descriptive statistics, including frequency, percentage, and range, were used to summarize and report the characteristics of SRs and prevalence of PIP. The characteristics of SRs are presented in Tables 1 and 2, and a narrative summary is provided. To show the distribution of the primary studies included in SRs by different geographical regions, the studies are shown as points on a globe map. A narrative approach was applied to synthesize heterogeneous data that were unsuitable for pooling.
RESULTS
1. Results of Literature Search and Study Selection
We identified 419 potentially relevant studies by searching the databases and other sources. All records were exported to Endnote X9 software (Clarivate). After eliminating duplicates (n=54), the titles and abstracts of 365 studies were screened, of which 270 irrelevant articles were excluded. Of the remaining articles, 95 full-text studies were assessed and 76 were excluded by reason. Finally, 19 SRs met our inclusion criteria for detailed analysis. The study selection process is shown in the Preferred Reporting Items for Systematic reviews and Meta-Analyses chart (Figure 1). A list of the excluded SRs is presented in Supplement 3.

Flow chart of the literature search and study selection process. PIP, potentially inappropriate prescribing.
Next, to present the prevalence of PIP in only community settings, a more detailed review of the primary studies (n=548) included in the 19 SRs was conducted, and those studies that were conducted in only community settings (n=311) were identified. The irrelevant primary studies (n=237), including those conducted on specific pathologies and/or medications, were excluded.
2. Characteristics of Included Systematic Reviews
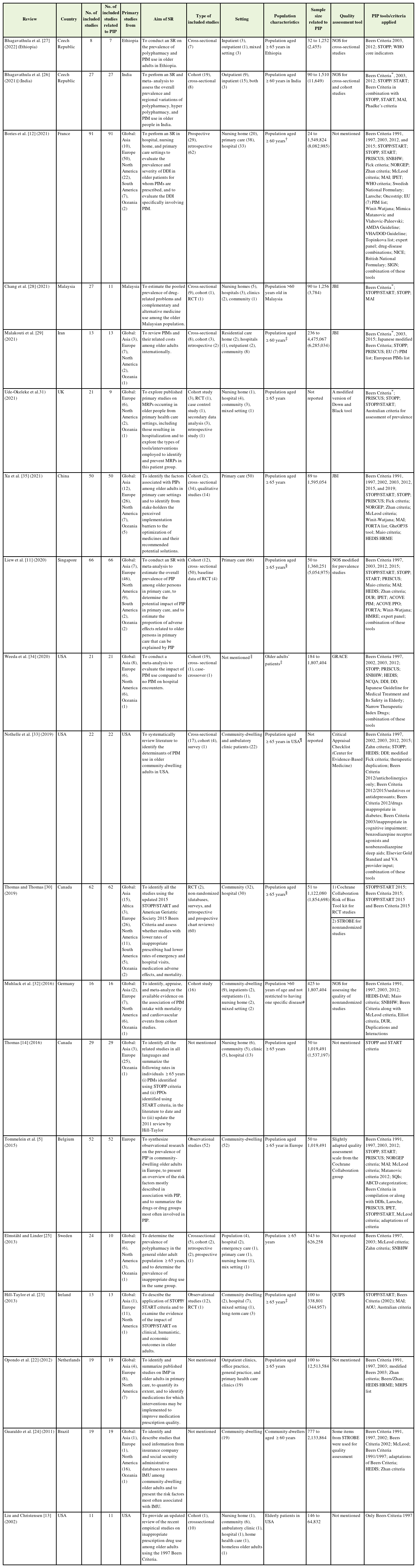
Characteristics of the 19 SRs are listed in Table 1. Most of the SRs were published after 2015 (n=14), including three articles from the United States, two from the Czech Republic and Canada, and one each from Brazil, China, Iran, Malaysia, Singapore, Belgium, France, Germany, Ireland, the Netherlands, Sweden, and the United Kingdom. The number of studies included in each SR ranged from 7 to 91, and the sample size of the primary studies ranged from 24 to 12,513,584. More than half of the primary studies were conducted in Europe (50.5%), followed by the United States (22.8%), and Asia (18.9%). Figure 2 shows the worldwide geographical distribution of primary studies on the prevalence of PIP. In 14 SRs, the estimation of prevalence of PIP was one of the main objectives [5,11-14,22-30]. In the remaining five SRs, the prevalence was reported in addition to the main outcomes [31-35].
3. Results of Quality and Overlap Assessment
The results of the methodological quality assessment of the SRs are provided in Supplement 4. Among the 19 SRs, seven SRs were classified as high quality [11,26-28,32,34,35], 10 as moderate [5,12,13,22-24,29-31,33], and two as low quality [14,25]. The median AMSTAR score was 5 (range, 3–11). The overall mean AMSTAR score of the included SRs was 6.36, which was considered to be of moderate quality.
The following points were highlighted in the AMSTAR items analysis: Only six SRs had published a prior protocol [11,26-28,33,35]. Publication status was used as the inclusion criterion for all SRs. Except four studies, none of the other SRs reported a list of excluded studies [13,26,27,32]. All 19 SRs provided information on the characteristics of the included studies. In 10 studies, the quality of the included primary studies was not properly assessed. Among these, five SRs did not assess the quality of the included studies [12-14,27,29]. Although the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was developed to improve the reporting of observational studies, two SRs used it to assess the risk of bias [26,30]. In two SRs, the quality assessment was not well documented [5,31]. In one SR, the risk of bias was not assessed for the cross-sectional studies that described the prevalence of PIP [23]. A list of the quality assessment tools used for each SR is presented in Table 1. Except for one, all SRs reported potential conflicts of interest [23].
The quality of evidence of the primary studies was reported by the review authors based on the judgment of the authors of SRs. In the nine SRs with an appropriate quality assessment, the quality of the included primary studies was reported to be high and moderate. Despite the lack of well-documented critical appraisal of the two SRs, the quality of their included studies was reported to be moderate and high [5,31].
In terms of overlap assessment, 413 out of the 548 included studies in the SRs were indexed publications (r=413), which resulted in a slight overlap, as estimated by a CCA of approximately 2%. A cross-table can be found in Supplement 5.
4. Prevalence of Potentially Inappropriate Prescribing in Community Settings
Due to the variety of applied criteria, lack of reporting of pooled effect size related to PIP by SRs, and methodological heterogeneity, a metaanalysis was not possible in this overview. Therefore, a narrative approach was applied.
As shown in Table 2, the prevalence of PIP derived from 311 primary studies that were only conducted in community settings had a wide range from 0% to 98%. In four SRs with mixed settings, the prevalence was reported according to setting [12-14,30]. Only in two SRs, the metaanalysis was conducted based on setting [26,28]. Of the 19 SRs, only six conducted a meta-analysis, of which two did not perform a metaanalysis on the prevalence of PIP [32,34]. In four SRs, the weighted mean prevalence was reported instead of the meta-analysis results (Table 2).
Only six SRs reported the prevalence of PIP according to these criteria [5,11,12,14,22,30]. For assessing PIP, the most widely used tool in community settings was Beers Criteria (all versions) (41.8%), followed by STOPP/START criteria (21.8%). Nineteen percent (n=60) of the primary studies used more than one screening tool, of which 16.7% of the studies used the Beers Criteria in compilation or along with one or more other criteria. Detailed information is presented in Table 2.
The most frequently reported PIP and/or medication classes were reported in nine SRs [5,13,14,22-24,29-31]. The most frequently reported PIPs are presented in Supplement 6. Narrative analysis of the results of SRs showed that benzodiazepines, nonsteroidal anti-inflammatory drugs, and antidepressants were the most frequently reported PIMs. Only four SRs reported the most frequently reported PPOs. Considering the wide range of PPOs reported by SRs, it was not possible to identify the most common PPOs.
DISCUSSION
This umbrella review aimed to map current literature related to the prevalence of PIP among community-dwelling older adults. This overview included 19 SRs comprising 548 primary studies. The overall prevalence of PIP in the community setting, determined using different criteria, showed a wide variation from 0% to 98%.
1. Prevalence of Potentially Inappropriate Prescribing
The range of prevalence of PIP derived from 311 primary studies conducted in a community setting was clearly identified. However, we attempted to provide an accurate prevalence of PIP by removing primary studies with different settings and studies conducted on specific pathologies and/or specific medications. However, much uncertainty remains regarding the extent of PIP in community settings. Several factors may contribute to these variations, including the diversity of screening methods used, different data sources, study populations, and diversity in healthcare systems across geographical regions.
2. Screening Methods
Approximately 30 different criteria were used in the 311 primary studies. Such variability in the tools used largely explains the variation in the prevalence range. A few SRs reported the prevalence of PIP by either criteria or conducted subgroup analyses by criteria. The prevalence differed significantly depending on the tool applied. In the study by Bories et al. [12], the significant difference observed between the Beers (38.6%) and the Norwegian General Practice (NORGEP) (26.0%) criteria was attributed to the fact that in the NORGEP criteria, inappropriate medications for specific comorbidities were not included. Moreover, the higher prevalence of PIP with the STOPP/START (60.4%) criteria results from the nature of this tool in the identification of underuse and overuse of medications [12]. The use of various tools to assess the prevalence of PIP in primary studies seems to be one of the reasons for the wide range of prevalence reported in different studies. Because of the differences in medication dosage and market availability, only a few medications are common to all PIP criteria [28].
Moreover, modifications made to the versions of the criteria may have contributed to the observed diversity. Although 41% of the primary studies used the Beers Criteria, different versions of the tool were applied across studies, and the overall rate of PIP was therefore not comparable between the studies. Since 2012, the Beers Criteria have been updated every 3 years. Some medications and/or medication classes have been either added or removed from the list [36], which seems to have had an impact on the diversity of the prevalence rates measured by various versions. Additionally, extensive adaptations of screening tools can explain this diversity [5]. Further, using adapted screening methods may have hampered the interpretation of the data resulting in the underestimation of reports on the true prevalence of PIP [5].
None of the PIP measures were superior to the others. It appears that the tools should be selected depending on the purpose, circumstances, and requirements of each case [37]. To enable comparisons between different tools and provide a more accurate and realistic picture of prevalence of PIP, it is suggested that SRs perform subgroup analyses using different criteria and/or limit their inclusion criteria to one or two specific tools as much as possible. Moreover, instead of using different adaptations of a tool, it is preferable to use a tool that is more compatible with the data collection method and region. For example, the use of GheOP3S (The Ghent Older People’s Prescriptions community Pharmacy Screening)-tool, and Matanovic criteria for limited clinical data in a European setting is a better approach. However, there are limited data on how well other criteria fit the prescribing situation in individual countries and regions [5], especially in countries other than those in Europe and America.
3. Geographical Locations
The quality of prescriptions, due to the diversity in healthcare systems across geographical locations, is another factor that may explain the difference in the observed prevalence across SRs. Because of registration issues, diverse sets of medications are used in different countries. Medication costs, the structure of medication financing, and reimbursement schemes of prescriptions can also affect the medication choices made in prescriptions. Furthermore, cultural differences in medication-related beliefs, attitudes, and practices found in various healthcare systems can overshadow the quality of prescriptions in older adults [38,39]. Future SRs should either focus on data from an individual country or conduct a subgroup analysis, if applicable, to minimize the effect of geographical location.
As more than half of the primary studies were conducted in European countries and the United States, we cannot generalize the reported prevalence to developing countries. A gap in studies in the field of prevalence of PIP can be observed in most Asian, South American, and African countries. This may have contributed to a lack of primary studies on SRs. There is wide diversity between countries in health system performance, medication-related beliefs, medication market availability, and different national databases. Therefore, to provide a comprehensive picture of the issue, there is a need for primary and SR studies in other countries, especially in low- and middle-income countries.
4. Data Source
Another reason for the diversity in prevalence may be the data sources used in these studies. The included SRs contained primary studies that relied on electronic medical records and/or secondary analyses of existing database sources such as the insurance databases and National Health Service databases. Insurance databases may not provide highquality documentation of clinical notes and may contain no details about medications and their usage [24]. However, they are generally considered as high-quality medication documentation because of their usefulness in reimbursement goals [24,40]. In countries where insurance companies do not have universal coverage, there is a risk of patient selection bias [22]. Compared to the provision of mixed information from any data source in SRs, the provision of separate information concerning prevalence of PIP, to health authorities by large administrative databases and/or data from insurance companies seems to present a better view.
5. Settings
In many cases, the majority of the population covered by administrative databases comprises community-dwelling older adults. In some SRs, however, this was not the case in truth. For example, in the SR conducted by Bories et al. [12], the setting of the primary study performed by Tora et al. [41] (they used data from patients with multidose drug dispensing [MDDD] services) was mentioned to be a community, while the majority of patients with MDDD lived in nursing homes. Health service, administrative, and insurance databases cover different populations in various countries. Therefore, primary studies should provide more detailed information regarding the databases used and their coverage. If the data include people from different settings, the prevalence rates should be reported separately.
6. Meta-analysis
A meta-analysis yields a more precise estimation of the outcome [42]. However, in the current review, we considered this inappropriate because of the significant heterogeneity that existed between the SRs and the primary studies they included. Only six SRs conducted meta-analyses [11,26-28,32,34]. In SRs without a meta-analysis, the exclusion of formal meta-analysis was attributed to significant heterogeneity in the populations, study design, care settings, measurement, and analysis methods (Table 2). Pooling studies that differ remarkably in their methods and other aspects produce meaningless results. Screening for the causes of heterogeneity among studies could also be advantageous. Subgroup analysis and meta-regression are two appropriate methods for investigating the reasons for heterogeneity [42]. Moreover, the authors of SRs can reduce potential sources of heterogeneity by controlling for the inclusion and exclusion criteria. It should be noted that inclusion criteria that are too broad can increase the heterogeneity between studies, while inclusion criteria that are too narrow make it difficult to find similar studies and may thus affect generalizability [43]. It is important to find a perfect balance when establishing the inclusion and exclusion criteria. However, the narrowness of the inclusion criteria to a particular setting, specific geographic area, and subgroup analysis can help avoid further sources of heterogeneity.
7. Quality of Studies
Although the quality of most of the included SRs was rated high or moderate, the quality of the primary studies was challenging. Two SRs inappropriately used the STROBE statement as a critical appraisal tool [24,30]. The misuse of the STROBE statement to assess the quality of studies is common in SRs. Da Costa et al. [44] reported that 53% of the SRs and meta-analyses inappropriately used STROBE.
However, in some SRs, the applied tool was not appropriate for quality assessment of prevalence studies. For instance, among SRs that used the Newcastle-Ottawa Scale (NOS), only one SR used the NOS modified for prevalence studies [11]. There are some tools that have been specifically developed for the risk of bias assessment of prevalence studies. It is recommended that further SRs examine the prevalence of PIP using validated tools proposed to assess prevalence studies. Notably, the Joanna Briggs Institute critical appraisal tool is recommended as a better tool for assessing the quality of prevalence studies, compared to others [45].
8. Most Frequently Reported Medications
Less than half of the SRs reported the most frequently reported medications. Regardless of the tool used, setting, or country, benzodiazepines were identified as the most frequently used PIP [46]. Because the most common medications may vary according to different settings, it is recommended that future SRs report the most common medication groups by setting. Regarding PPO, we could not report the most frequently omitted medications because of the small number of SRs that reported them. Presenting the most frequently prescribed medications separately using PIM and PPO may represent a more accurate image of PIP.
9. Strengths and Weaknesses
This overview presents a comprehensive and global view of SRs to inform and increase the value of future research, by including 19 SRs containing more than 500 primary studies. We identified the regions where fewer studies have been conducted on the prevalence of PIP. Although some SRs did not pay attention to the differences in study settings and their effects on the prevalence of PIP, this overview attempts to provide a better view of the PIP situation among community-dwelling older adults.
Our study has some limitations that deserve consideration. One limitation was the wide range of prevalence that originated from the wide variation in estimates in the included SRs. This variation limits the generalizability of the study findings. Additionally, the significant heterogeneity identified between the SRs and the low number of SRs with meta-analysis of the studies that were performed in community settings made it impossible for us to conduct a meta-analysis in the present study. The purpose of an umbrella review is not only to present a pooled effect size, but also to map the available evidence, summarize and compare the results of several SRs, identify gaps in the literature, and highlight the sources of conflicting results. Therefore, our findings in the present umbrella review can offer an opportunity for decision makers and researchers to base their decisions on the best evidence according to their situation [47,48].
10. Implications for Practice
Frequent assessment of PIPs in community-dwelling older adults can prevent the development of adverse health outcomes such as hospitalization. Considering the distribution of primary studies on the prevalence of PIP, the importance of developing continuous monitoring and registration systems concerning PIP status is highlighted, especially in developing and low-income countries without suitable recording and monitoring systems.
CONCLUSION
This study provides a comprehensive view of the status of existing SRs related to the prevalence of PIP and the implications for improving the evidence base in both future prevalence studies and SRs. This overview of SRs revealed considerable variations in the prevalence of PIP in community settings. Not surprisingly, the large amount of heterogeneity between SRs and their included primary studies precluded us from providing an accurate estimation of the prevalence of PIP among community-dwelling older adults. Similar to the findings reported by Borges Migliavaca et al. [45], heterogeneity was identified as the main challenge in our study, which may be due to the wide range of accepted critical appraisal tools. To help identify the knowledge-to-action gaps, SR authors should consider the differences in the prevalence of PIP by setting, applied tools, data sources, geographical areas, and specific pathology, and pay more attention to proper quality assessment while planning for developing SRs. Moreover, finding a perfect balance between establishing the inclusion and exclusion criteria in future SRs might be helpful in controlling potential heterogeneity. Since more than half of the primary studies included in the 19 SRs were conducted in European and American countries, a knowledge gap in Asian, African, and Latin American countries in terms of prevalence of PIP is obvious. There is a need for primary and SR studies from other countries, especially low- and middle-income countries. This overview highlights the need to develop monitoring and recording systems in developing and low-income countries. Providing a more accurate assessment of the extent of PIP can help health policymakers establish high-quality monitoring systems to reduce PIP in their healthcare systems and provide high-quality care.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Hereby, we thank the Research Deputy of Tabriz University of Medical Sciences, the Faculty of Health Science.
Notes
FUNDING
This research was funded by Tabriz University of Medical Sciences (Grant no., 68506). The funding center had no role in the design, analysis, or writing of this article.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.23.0032.
PRIOR checklist.
Detailed search strategy.
Excluded articles with reason.
AMSTAR.
Overlapping among systematic reviews.
The most frequently reported PIPs or medication classes reported in systematic reviews.