 |
 |
- Search
| Korean J Fam Med > Volume 45(2); 2024 > Article |
|
Abstract
We report a rare case of high-volume training-related myopericarditis. A male, 18 years old, elite road bicycle racing cyclist with high-volume training of 1,000 km per week for >7 years, presented with progressively worsening exertional breathlessness, reduced effort tolerance, and one episode of cardiac syncope. The symptoms were present prior to the coronavirus disease 2019 pandemic but made worse with the sudden increase in the volume of training after lockdown periods in preparation for competition. He exhibited multiple premature ventricular ectopic beats during his resting electrocardiogram, with a normal echocardiogram and non-elevated cardiac enzyme. The exercise stress test revealed similar multiple premature ventricular beats, warranting further investigation using cardiac magnetic resonance imaging (MRI). The findings of the cardiac MRI were suggestive of myopericarditis. He was instructed to refrain from training and initially started with a short course of colchicine. However, his symptoms deteriorated, and cardiac MRI revealed a decrease in the left ventricular ejection fraction from 59% to 50%. His treatment was escalated to a short course of tapered dose steroid, anti-failure medication and gradual, supervised, return to sports program. This case report highlights the discussion of return to play in athletes with myopericarditis.
Heart disease and sudden cardiac death (SCD) in athletes are rare but can result in dramatic and unexpected emergency events. This attracts considerable media attention as athletes are often young and viewed as a role model for good physical fitness and health. Although the incidence of SCD is low (1 in 40,000 to 1 in 80,000 athletes per year), aggressive preventive strategies and screening should be implemented in athletes prior to sports participation to prevent such events [1]. In young athletes less than 35 years old, SCD is commonly caused by hypertrophic cardiomyopathy and anomalous events in a coronary artery [2]. A total of one in three athletes with heart disease will report cardiac symptoms, which include exertional chest pain, extreme shortness of breath during exertion, reduced fitness and performance, palpitations, pre-syncope, or syncope [2]. Thus, athletes with these symptoms should have an extensive cardiac work up before clearance to participate in sporting events. To the best of our knowledge, this is the first report to describe the incidence of myopericarditis owing to high-intensity training in an elite road racing cyclist. Our objective was to report a rare occurrence of high-volume training-related myopericarditis and outline the management and return to play of athletes with such condition.
This is a case of a male, 18-year-old, national road racing cyclist, who presented to clinic with history of progressively worsening exertional breathlessness associated with exertional chest pain and one episode of exertional syncope. He developed these symptoms in November 2020, but the symptoms remained mild to nonexistent during the lockdown period in the coronavirus disease 2019 (COVID-19) pandemic. He started to develop worsening of symptoms once he restarted his training post lockdown, with a sudden increase in the intensity and volume of training. The presence of syncope worried him and led to the referral to the sports medicine clinic at the University Malaya Medical Centre, Kuala Lumpur, Malaysia, in January 2022. He is an elite road racing cyclist with a minimum training distance of 1,000–1,500 km cycling per week for the past 7 years and had a dramatic increase in volume and intensity to 2,000 km per week a few months prior to the referral to the sports clinic. He is a non-smoker and does not have any risk factors of developing cardiovascular disease. He denies any ingestion of off-label supplements or medications but reported high ingestion of caffeine tablets (400 mg per day) for 1 year under supervision of a nutritionist. He denies any underlying medical condition, history of COVID-19 infection, autoimmune symptoms, or hypo/hyperthyroid symptoms. During the first presentation in November 2020, he denied any episodes of fever or illness prior to the onset of symptoms. He received the Pfizer messenger RNA COVID-19 vaccine on May 17, 2021 (EX6564) and June 7, 2021 (FC3558), but the symptoms started at the end of November 2020, prior to the pandemic. Clinical examination revealed normal vital signs, and normal cardiovascular examinations with no sign of heart failure.
Electrocardiogram (ECG) revealed a sinus rhythm with features of athletic hearts; left ventricular hypertrophy, and interventricular conduction delays. However, there were abnormal findings of multiple left sided premature ventricular complexes. He underwent an exercise stress test (EST) and was only able to complete up to stage 3 owing to shortness of breath. EST revealed multiple premature ventricular complexes at rest, during exercise, and during recovery. His blood pressure showed a good response to exercise, with no significant hemodynamic instability. Ideally, a Holter examination should be performed to investigate the burden of premature ventricular contractions (PVCs), but was not available at our center during the period of assessments. His echocardiogram revealed a normal wall thickness with ejection fraction of 60%. Cardiac magnetic resonance imaging (MRI) indicated myopericarditis, revealed a non-ischemic epicardial enhancement at basal to mid-inferior wall, mild pericardial effusion with systolic function (59%), and normal wall thickness (inter-ventricular septum thickness in diastole 9 mm) (Figure 1). Surprisingly, troponin I, cardiac markers, and inflammatory markers were not raised.
As the MRI revealed myopericarditis, he was treated with colchicine for 3 months and refrained from any form of training for 3 months. However, after 3 months of treatment, the symptoms worsened. Repeated cardiac MRI revealed a decline in ejection fraction from 59% to 50% (Figure 2). The treatment was escalated to a tapered course of prednisolone for 3 months with anti-failure medication and colchicine. The patient’s symptoms improved after steroid therapy. A summary of his progress is presented in Table 1.
The decision to return to cycling was made based on a shared decision approach. As his burden of PVCs improved as well as symptoms with no life-threatening arrhythmias during the EST, he was allowed to gradually return to sports. The patient’s return to the sports program is shown in Table 2. The key point of progressing his return to play was based on the absence of symptoms during activity and rest, no lifethreatening changes during EST, and normal left ventricular function. We did not monitor his cardiac enzymes as they were normal. After 6 months of cessation of training, and 18 months after initially presenting, he returned to sports and was able to return to competition. Repeat MRI revealed resolution of myopericarditis, with an ejection fraction of 68% and left ventricular dysfunction. However, we recommended that the patient have adequate rest periods in a week and reduced volume of training. Furthermore, basic life support care was given to his supporting staff to ensure a safe return to sports. We also advised that automated external defibrillator should be always present during his training and competition. Given the risk of recurrence, comprehensive yearly cardiac assessments will also be conducted.
The patient provided written informed consent for the publication of the research details and clinical images.
Myopericarditis is a new term describing pericarditis with myocardial involvement when there is primarily pericarditis evidenced by cardiac biomarker elevation or imaging studies revealing edema and late gadolinium enhancement with normal wall motion and no left ventricular systolic dysfunction [3,4]. Our case demonstrates that high-volume training can cause myopericarditis. A common presentation of myopericarditis is chest pain associated with ST elevation in ECG and raised cardiac troponin [3,5]. Thus, in this presentation, a cardiologist would perform angiography to exclude any coronary event. While the most common cause of myocarditis is viral such as Epstein-Barr virus, limited data suggest myocarditis can be triggered by overtraining [6]. Lara et al. [7] in 2019 observed a significant myocardial stress following long distance endurance exercise, marked by significant elevated cardiac enzymes and pro-B-type natriuretic peptide. Another study also demonstrated significant myocardial stress following competitive exercise in professional road cyclists, but no abnormal structural heart disease was detected during cardiac assessments [8]. Despite the weak link between overtraining and myocardial injury, we believe our case is likely training related as there were no symptoms of infection prior to the event, and the symptoms progressed very slowly over years, which is different from typical viral myocarditis. Overtraining can cause immunosuppression, which predisposes athletes to possible viral infection, which could lead to subclinical myocarditis in the initial phase [6].
Cardiac troponins are well established as sensitive and specific markers of myocardial injury resulting from myocarditis [3,5]. However, in a few isolated cases, they can be normal, as in our case. ECG of myopericarditis often has similar features to pericarditis ECG. The stages of ECG changes are well documented for acute pericarditis, from the presence of diffuse ST segment elevation to T-wave inversion to normalization of the ECG [3]. In our case, the athlete did not present a typical ECG for myopericarditis, possibly because of normalization of the ECG. However, the presence of PVCs was suggestive of myocardial scarring. A normal ECG with normal cardiac enzymes does not exclude the presence of myopericarditis [9].
Cardiac MRI is a good modality for diagnosing myopericarditis, with a sensitivity and specificity of 86% and 95%, respectively [3,5,10]. The presence of edema and/or inflammation in pericardium and myocardium with normal systolic function is suggestive of myopericarditis [5]. This is best viewed in late gadolinium T2 imaging, high-intensity signals at global or regional in myocardial T2 relaxation time, or in myocardial signal intensity in T2-weighted images. Biopsy is not usually indicated, and reserved for patients who develop worsening heart failure despite optimum therapy [5]. Left ventricular dysfunction should be monitored as it carries high prognostic value [5]. The prognosis of myopericarditis is good, with 50% reporting a resolution, 25% exhibiting persistent cardiac dysfunction, and 15%–25% developing fulminant heart failure [5].
Studies to guide the management of myopericarditis are limited. When myopericarditis presents with preserved ventricular function without the presence of significant ventricular arrhythmias, it is managed in a similar fashion to acute pericarditis, which primarily involves the use of a short course of high-dose nonsteroidal anti-inflammatory drugs (NSAIDs) or colchicine [3,5,11]. However, in several animal models, the use of NSAIDs in the setting of myocardial inflammation has a detrimental effect, increasing mortality rates and possibly exacerbating the myocarditis process [3,11]. At this time, there is no conclusive data that the use of colchicine provides any significant benefit in the management of myopericarditis. However, it is often used on a case-by-case basis in clinical practice. Raval et al. [12] in 2015 conducted a meta-analysis on the role of colchicine in pericarditis and established it is effective in preventing both primary and recurrent episodes of pericarditis, and has tolerable side effects. Thus, we opted for colchicine in our case because it has a better safety profile than NSAIDs over the long course of treatment. The use of beta blockers and angiotensin-converting enzyme-inhibitors is advocated in the setting of global and/or regional left ventricular dysfunction [13]. Beta blockers can be used as an adjunct to control symptoms such as palpitations and chest pain [3,11]. Steroids should be used with caution given the relative lack of evidence, and should only be considered in an autoimmune condition or non-responsive to first line therapy as in our case [3,11].
The key point of this report is its description of the management of return to play in an athlete who has myopericarditis based on 2020 European Society of Cardiology guidelines [5]. For the first 3–6 months during treatment, athletes should refrain from any competitive sports and moderate to high-intensity training [5]. This is to remove the stressors and allows the inflammation to subside. However, earlier return to sports is possible guided by the symptoms and resolution of edema in MRI [5]. Repeat assessments, including ECG, EST, Holter, cardiac biomarkers, and cardiac MRI, should be performed after the resting period. Ideally, the criteria for return to sports in athletes with myopericarditis are [5]: (1) normalization of left ventricular function, wall motion, and cardiac dimensions based on echocardiogram or radionuclide study; (2) clinically relevant arrhythmias, such as frequent and/or complex repetitive forms of ventricular or supraventricular ectopic activity, are absent on ambulatory Holter monitoring and graded exercise testing; (3) normalization of cardiac markers and brain natriuretic peptide; and (4) normalization of 12 lead ECG.
Persistent myocardial scarring >20% or presence of myocardial dysfunction is a contraindication to return to sports [5]. Athletes with myopericarditis should have a gradual return to play (Table 2) and ensure that in each stage, there are no symptoms such as chest pain, palpitation, syncope, or exertional breathlessness. However, a shared decision-making approach on a case-by-case basis should be adopted by athletes, physicians, coaches, and other important supporting staff because there is always an increased risk of cardiac complications, including SCD, in the future.
In conclusion, myopericarditis related to high training levels is rare. Compared to other noninvasive investigations, cardiac MRI is a good modality in diagnosis myopericarditis. Return to play of an athlete with myopericarditis should be gradual and they should be asymptomatic, with loss of abnormal ECG findings, normal left ventricular function, regional wall motion, and cardiac dimension, and resolution of myopericarditis or scar in cardiac MRI.
Figure. 1.
First cardiac magnetic resonance imaging images. (A) Enhancement at the inferior wall of the left ventricle (white arrow). (B, C) Nonischemic epicardial enhancement in the late gadolinium study at the basal to midinferior wall (yellow arrows). The patient’s ejection fraction was 59%.
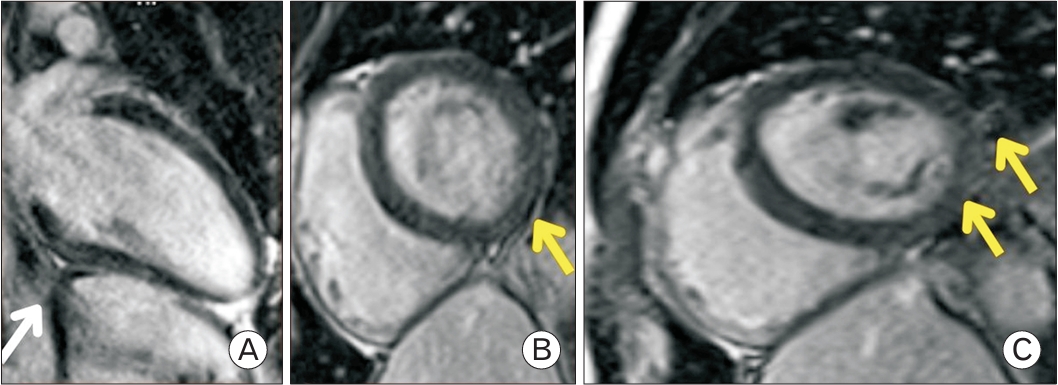
Figure. 2.
Repeated cardiac magnetic resonance imaging (MRI) performed after 3 months. (A) Shows pericardial effusion, which was not observed in the previous MRI (white arrow). (B) There is a reduction in epicardial enhancement on the previous MRI scan (yellow arrow). (C) However, the current MRI demonstrates a reduction in the ejection fraction to 50% (yellow arrow). AHR, adjusted hazard ratio.
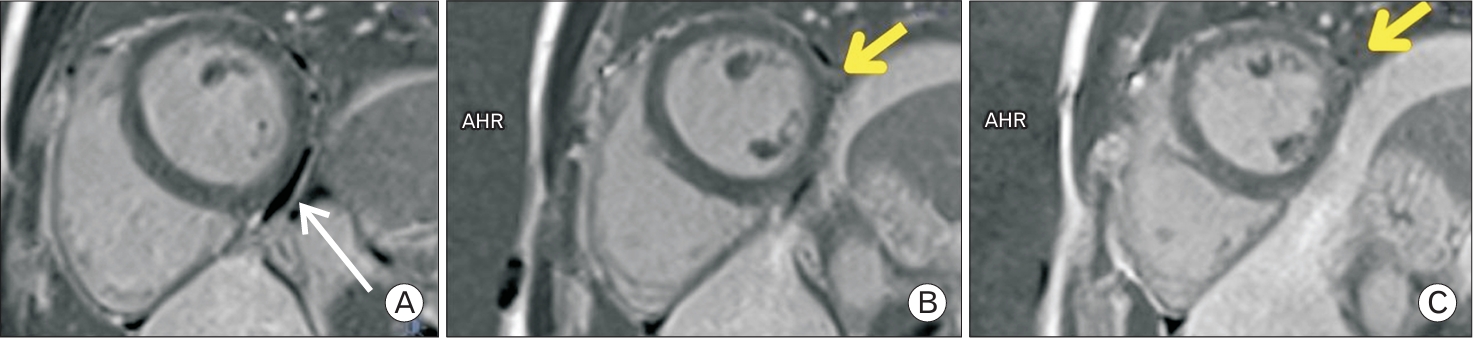
Table 1.
Athlete’s progress from first follow-up until return to play
Table 1 demonstrate the progress of our athlete from the first FU until his RTP. We monitored his symptoms, echocardiogram, and EST prior to progress of his RTP.
FU, follow-up; NYHA, New York Heart Association; Echo, echocardiogram; LVEF, left ventricular ejection fraction; LVSF, left ventricular systolic function; LVDF, left ventricular diastolic function; EST, exercise stress test; PVC, premature ventricular contractions; CMR, cardiac MRI; RTP, return to play.
Table 2.
Proposed guideline for return to play in athlete with myopericarditis
REFERENCES
1. Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Heart 2014;100:1227-34.


2. Wasfy MM, Hutter AM, Weiner RB. Sudden cardiac death in athletes. Methodist Debakey Cardiovasc J 2016;12:76-80.



3. Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol 2008;127:17-26.


4. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, Savorgnan F, Guggilla RK, Khuri-Bulos N, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022;40:1499-511.


5. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Rev Esp Cardiol (Engl Ed) 2021;74:545.

6. Halle M, Binzenhofer L, Mahrholdt H, Johannes Schindler M, Esefeld K, Tschope C. Myocarditis in athletes: a clinical perspective. Eur J Prev Cardiol 2021;28:1050-7.



7. Lara B, Salinero JJ, Gallo-Salazar C, Areces F, Ruiz-Vicente D, Martinez M, et al. Elevation of cardiac troponins after endurance running competitions. Circulation 2019;139:709-11.


8. Konig D, Schumacher YO, Heinrich L, Schmid A, Berg A, Dickhuth HH. Myocardial stress after competitive exercise in professional road cyclists. Med Sci Sports Exerc 2003;35:1679-83.


9. Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 2007;28:1242-9.


10. Liu PP, Yan AT. Cardiovascular magnetic resonance for the diagnosis of acute myocarditis: prospects for detecting myocardial inflammation. J Am Coll Cardiol 2005;45:1823-5.








