 |
 |
- Search
| Korean J Fam Med > Volume 42(3); 2021 > Article |
|
Abstract
Background
Methods
Results
Figure. 1.
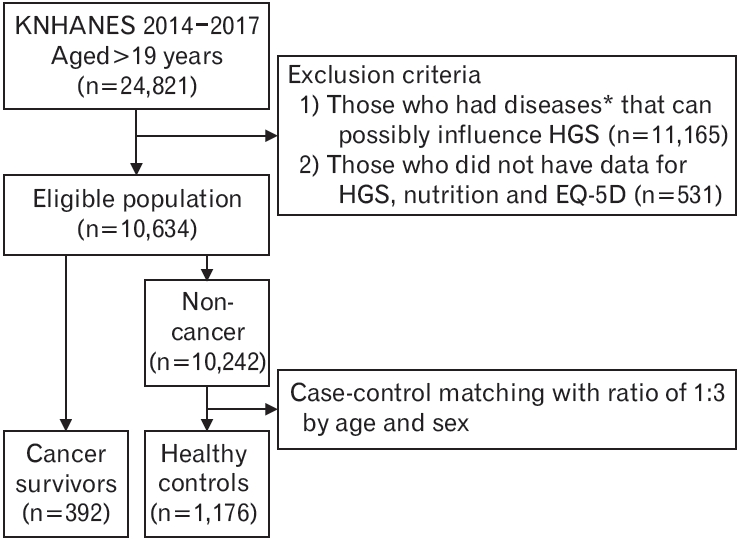
Table 1.
| Characteristic |
Male (n=512) |
Female (n=1,056) |
|||||
|---|---|---|---|---|---|---|---|
| Cancer survivors (n=128) | Healthy controls (n=384) | P-value | Cancer survivors (n=264) | Healthy controls (n=792) | P-value | ||
| Age (y) | 59.47±1.87 | 58.97±0.88 | 0.801 | 51.40±0.73 | 50.42±0.46 | 0.254 | |
| Household income | 0.489 | 0.460 | |||||
| Low | 19.4 (3.8) | 21.5 (2.3) | 9.0 (1.7) | 10.5 (1.1) | |||
| Low-mid | 27.8 (4.4) | 20.8 (2.3) | 23.7 (2.9) | 25.3 (1.9) | |||
| Mid-high | 24.0 (4.9) | 29.9 (2.9) | 33.9 (3.4) | 28.3 (1.8) | |||
| High | 28.8 (5.3) | 27.8 (2.7) | 33.4 (3.6) | 35.9 (2.2) | |||
| Education | |||||||
| ≤High school graduate or less | 66.5 (5.3) | 61.8 (3.0) | 0.431 | 67.1 (3.4) | 65.4 (2.2) | 0.665 | |
| Live alone | 7.3 (2.5) | 9.2 (1.6) | 0.540 | 6.1 (1.3) | 6.1 (0.8) | 0.962 | |
| Cancer | |||||||
| Gastric cancer | 36.2 (4.8) | 9.9 (2.1) | |||||
| Liver cancer | 4.4 (1.8) | 0.2 (0.2) | |||||
| Colon cancer | 16.4 (3.6) | 6.7 (1.8) | |||||
| Breast cancer | - | 20.9 (2.9) | |||||
| Cervical cancer | - | 19.4 (2.8) | |||||
| Lung cancer | 7.9 (3.1) | 1.3 (0.8) | |||||
| Thyroid cancer | 6.0 (2.4) | 31.3 (3.2) | |||||
| Others | 35.6(5.1) | 14.0 (2.4) | |||||
| Time since diagnosis ≤5 y | 61.5 (5.1) | 57.8 (3.4) | |||||
| Aerobic physical activity | 49.8 (5.3) | 51.0 (3.0) | 0.843 | 54.1 (3.9) | 46.6 (2.1) | 0.089 | |
| Resistance exercise ≥2 d/wk | 27.2 (4.4) | 30.4 (2.7) | 0.549 | 19.0 (2.7) | 13.9 (1.4) | 0.082 | |
| Body mass index (kg/m2) | 22.89±0.38 | 23.55±0.19 | 0.118 | 22.59±0.20 | 22.77±0.13 | 0.443 | |
| Total caloric intake (kcal/d) | 2,092.97±109.65 | 2,255.56±82.50 | 0.224 | 1,706.54±52.97 | 1,771.44±30.69 | 0.305 | |
| Total protein intake (g/d) | 79.36±6.45 | 85.25±8.31 | 0.572 | 60.09±2.02 | 62.86±1.31 | 0.253 | |
| At risk drinking* | 38.6 (6.7) | 50.4 (3.5) | 0.132 | 19.2 (3.9) | 26.2 (2.2) | 0.140 | |
| Smoking | 0.056 | 0.922 | |||||
| Current smoker | 17.1 (4.1) | 30.3 (2.9) | 4.0 (1.4) | 3.9 (0.8) | |||
| Ex-smoker | 60.3 (5.1) | 48.8 (2.9) | 4.0 (1.3) | 4.6 (0.9) | |||
| Non smoker | 22.6 (4.6) | 20.8 (2.3) | 92.0 (1.9) | 91.5 (1.2) | |||
| Hand grip strength in dominant hand (kg) | 34.91±1.20 | 36.76±0.66 | 0.165 | 22.97±0.63 | 23.31±0.31 | 0.631 | |
| Subjects with low hand grip strength† (%) | 17.2 (3.6) | 16.0 (2.0) | 0.749 | 12.8 (2.6) | 11.2 (1.3) | 0.569 | |
Values are presented as estimated mean±SE or estimated % (SE). P-values were for the complex samples cross tabulation for categorical variables and the complex samples general linear model for continuous variables. A P-value of less 0.05 was considered as statistically significant.
SE, standard error.
Table 2.
| EQ-5D |
Male (n=512) |
Female (n=1,056) |
||||
|---|---|---|---|---|---|---|
| Cancer survivors (n=128) | Healthy controls (n=384) | P-value | Cancer survivors (n=264) | Healthy controls (n=792) | P-value | |
| Mobility: have problems* | 14.8 (3.4) | 9.9 (1.7) | 0.153 | 7.9 (1.9) | 7.3 (1.0) | 0.784 |
| Self-care: have problems* | 5.7 (2.3) | 1.5 (0.6) | 0.011 | 2.2 (0.9) | 1.7 (0.5) | 0.611 |
| Usual activities: have problems* | 11.6 (3.6) | 4.8 (1.2) | 0.023 | 7.5 (1.9) | 2.9 (0.6) | 0.005 |
| Pain/discomfort: have problems* | 24.6 (4.6) | 15.6 (2.2) | 0.059 | 22.8 (2.9) | 20.7 (1.7) | 0.537 |
| Anxiety/depression: have problems* | 8.3 (2.9) | 8.1 (1.8) | 0.952 | 14.3 (2.9) | 7.6 (1.0) | 0.011 |
| EQ-5D index | 0.94±0.01 | 0.96±0.05 | 0.030 | 0.95±0.07 | 0.96±0.03 | 0.053 |
Table 3.
| EQ-5D |
Cancer survivors (n=128) |
Healthy controls (n=384) |
P for interaction‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Crude |
Multi-adjusted† |
Crude |
Multi-adjusted† |
|||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Problem in mobility§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 4.59 (1.41–14.98) | 0.012 | 2.09 (0.61–7.16) | 0.237 | 4.65 (2.19–9.87) | <0.001 | 1.78 (0.65–4.87) | 0.260 | 0.905 | |
| Problem in self-care§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 7.07 (1.30–38.59) | 0.024 | 8.51 (1.69–42.83) | 0.010 | 13.53 (1.56–117.14) | 0.018 | 2.25 (0.29–17.55) | 0.438 | 0.643 | |
| Problem in usual activities§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 3.70 (0.91–15.11) | 0.068 | 6.63 (1.22–36.03) | 0.029 | 5.89 (2.11–16.45) | 0.001 | 2.47 (0.56–10.87) | 0.230 | 0.918 | |
| Pain/discomfort§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 1.63 (0.51–5.17) | 0.408 | 2.67 (0.90–8.00) | 0.077 | 2.29 (1.14–4.62) | 0.020 | 1.33 (0.54–3.30) | 0.532 | 0.923 | |
| Anxiety/depression§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00C | ||||||
| Low HGS* | 3.88 (0.88–17.17) | 0.074 | 2.73 (0.44–17.14) | 0.280 | 1.64 (0.62–4.30) | 0.318 | 0.98 (0.37–2.60) | 0.962 | 0.482 | |
OR, odds ratio; CI, confidence interval; QOL, quality of life; HGS, hand grip strength; EQ-5D, European Quality of Life Scale-Five Dimensions.
* The cut-off value for low HGS was 28.9 kg in men. There were 102 men with normal HGS and 26 men with low HGS among cancer survivors. In the healthy controls, there were 303 men with normal HGS and 81 men with low HGS.
Table 4.
| EQ-5D |
Cancer survivors (n=264) |
Healthy controls (n=792) |
P for interaction‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Crude |
Multi-adjusted† |
Crude |
Multi-adjusted† |
|||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Problem in mobility§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 7.00 (2.21–22.20) | 0.001 | 5.87 (2.04–16.91) | 0.001 | 2.80 (1.45–5.44) | 0.002 | 1.06 (0.50–2.22) | 0.888 | 0.079 | |
| Problem in self-care§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 7.01 (1.24–39.52) | 0.028 | 8.85 (0.49–158.37) | 0.138 | 4.61 (1.50–14.20) | 0.008 | 1.49 (0.26–8.46) | 0.656 | 0.438 | |
| Problem in usual activities§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 11.44 (3.43–38.18) | <0.001 | 14.46 (3.84–54.44) | <0.001 | 4.08 (1.64–10.15) | 0.003 | 2.40 (0.80–7.19) | 0.117 | 0.061 | |
| Pain/discomfort§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 4.53 (1.79–11.46) | 0.001 | 4.90 (2.00–12.01) | 0.001 | 1.30 (0.75–2.25) | 0.354 | 1.20 (0.64–2.24) | 0.571 | 0.004 | |
| Anxiety/depression§ | ||||||||||
| Normal HGS | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Low HGS* | 6.87 (2.43–19.37) | <0.001 | 6.43 (2.16–19.12) | 0.001 | 1.38 (0.68–2.81) | 0.371 | 1.00 (0.45–2.23) | 0.998 | 0.024 | |
OR, odds ratio; CI, confidence interval; QOL, quality of life; HGS, hand grip strength; EQ-5D, European Quality of Life Scale-Five Dimensions.
* The cut-off value for low HGS was 16.8 kg women. There were 234 women with normal HGS and 30 women with low HGS among cancer survivors. In the healthy controls, there were 693 women with normal HGS and 99 women with low HGS.
REFERENCES
- TOOLS







